Mineral fortification systems
a technology of mineral fortification and mineral fortification, which is applied in the field of mineral fortification systems, can solve the problems of significant distribution logistics problems, insufficient levels of necessary minerals and nutrients in the average diet, and high cost of providing minerals and vitamins as a supplement, and achieves the effect of acceptable tas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0099] A mineral fortification powder is prepared having the following ingredients in the indicated amounts:
IngredientAmountSunActive Iron (8.0% Fe)1.8mgZinc bis-glycinate (21.8% Zn)1.5mgVitamin C as sodium ascorbate (88.9%60mgVit. C)Vitamin B60.2mgVitamin B12 (1% Vit. B12)0.6microgmCitric Acid.01gmFolic Acid40microgm
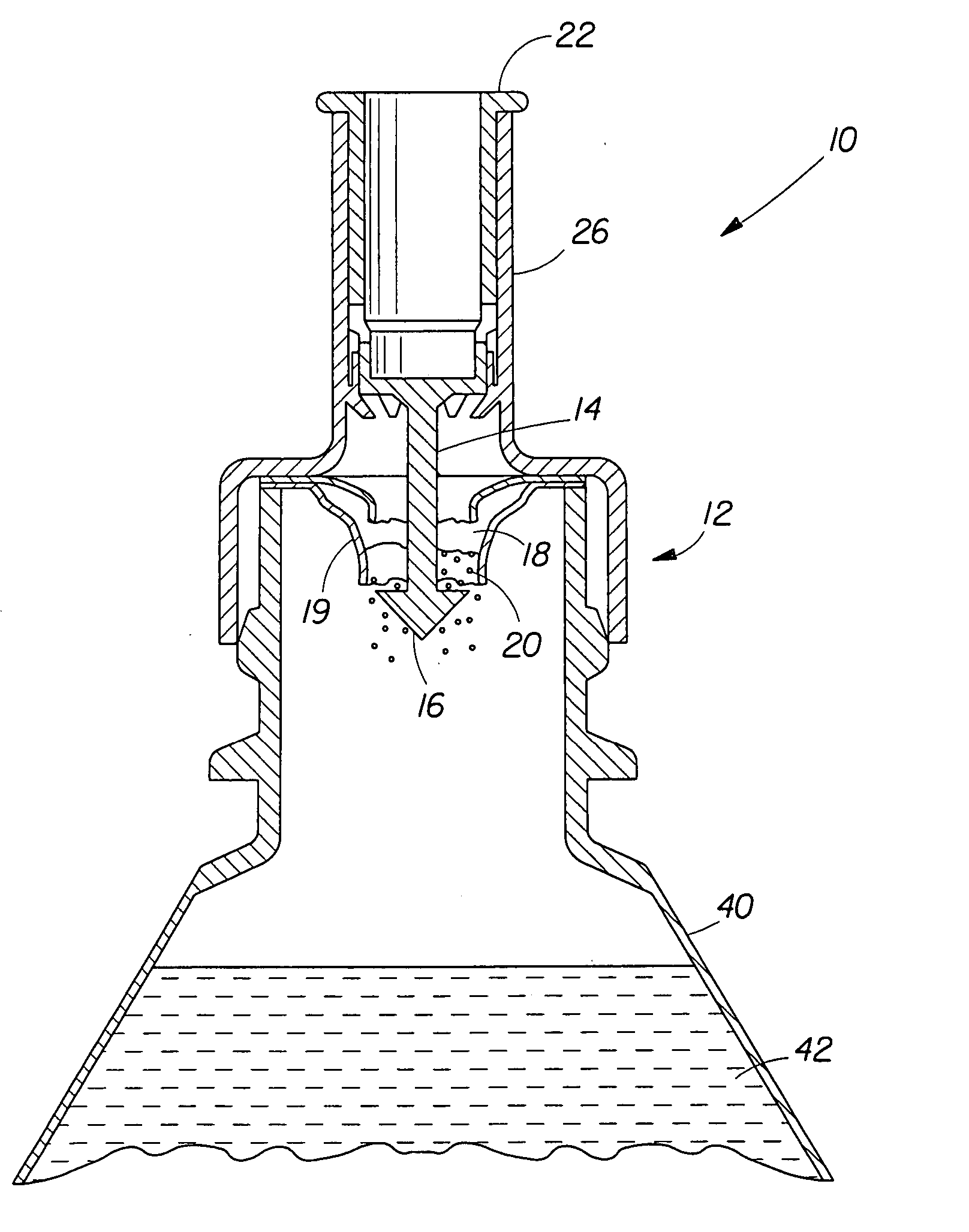
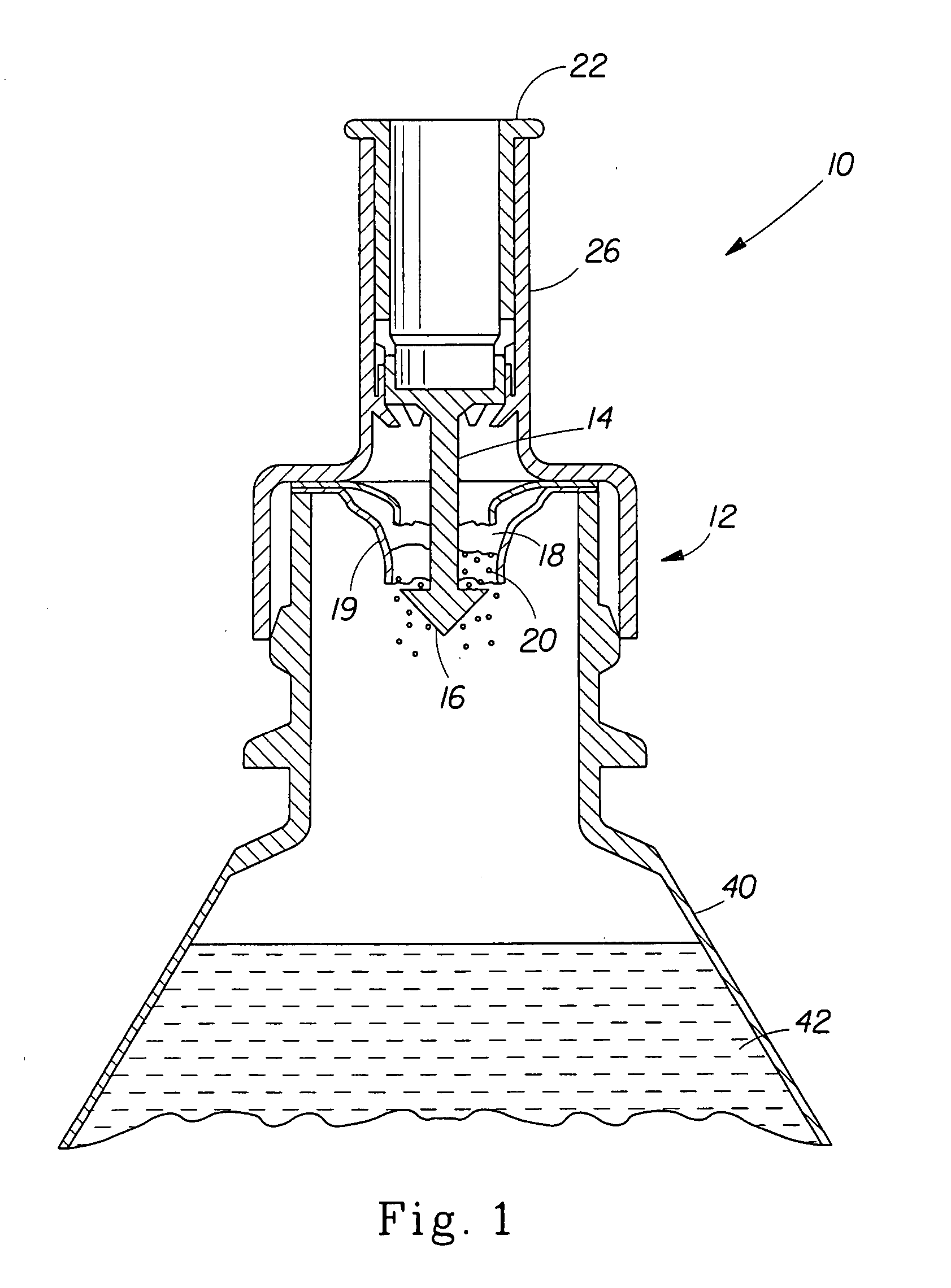
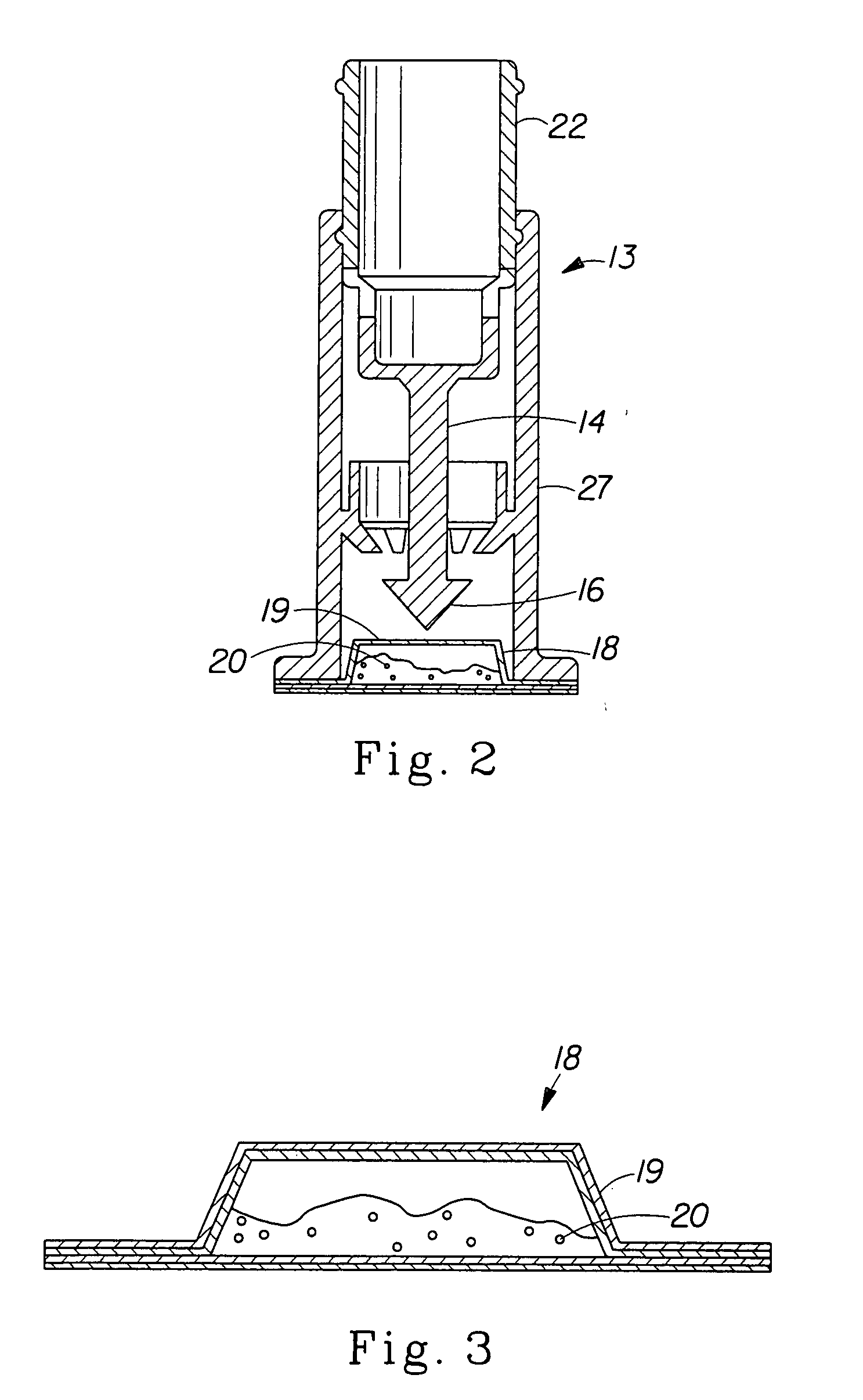
[0100] Upon preparing the mineral fortification powder, it is placed in a sealed pouch and inserted into a cap as shown in FIG. 1. The cap is then placed on a bottle of Reverse Osmosis / Millipore (Milli-Q) water and the plunger is depressed. The bottle of water is gently swirled to mix the powder and to make a fortified liquid composition that has no off-color or rusty color, no precipitation or turbidity, and low redox potential. The taste of the liquid composition is not significantly different in metallic taste or after-taste when compared to the liquid vehicle alone (Reverse Osmosis / Millipore (Milli-Q) Water).
example 2
[0101] A mineral-fortified liquid composition according to the present invention, and more specifically, according to Example 1, was compared to common tap water, distilled water treated by a common Reverse Osmosis process, and a variety of commercially available bottle waters. Some of the commercially available bottled waters were supplemented with vitamins. Using the measure values for the Redox potential (listed as “mV” in Table 2A) and pH, the inequality 0≧RP−(A−B*pH) was calculated for various values of “A” and “B”. The results of these calculations are given in Table 2A. Table 2B gives additional data from the comparison of these products.
TABLE 2AA = 400A = 380A = 360A = 340mVPHB = 20B = 18B = 16B = 14Water of Example 11924.85−111−101−90−80Tap water3168.95959799101Reverse Osmosis3605.75758492101waterFresh milliQ13206.5250576471Stored milliQ3365.7451596876Aquafina Plus4034.218799110122Calcium2Aquafina Multi-V3653.9644566880Aquafina Daily C3384.0419314355Hansen Energy34063.768...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com