Pre-Clinical Animal Testing Method
a pre-clinical animal and testing method technology, applied in the field of pre-clinical animal testing methods, can solve the problems of inability to replace an implanted device, the conventional power supply of an implantable device may not be suitable for a variety of implanted devices, and the size of an implantable drug delivery device such as a pump may be too large for many locations in the body, so as to facilitate the disintegration of the implanted devi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Chemical Releasing Microchip with Electrochemical Actuation and RF Power Transmission
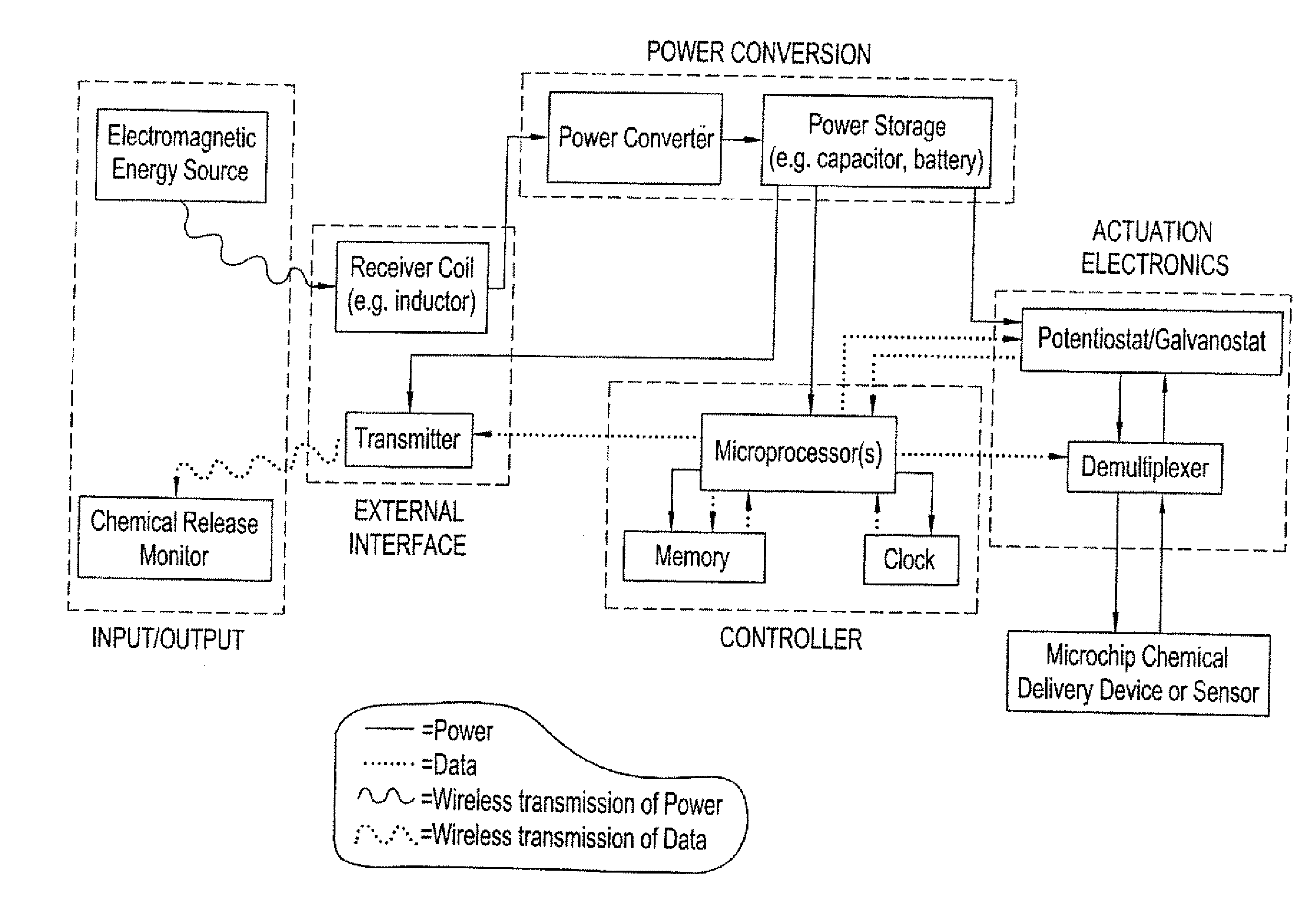
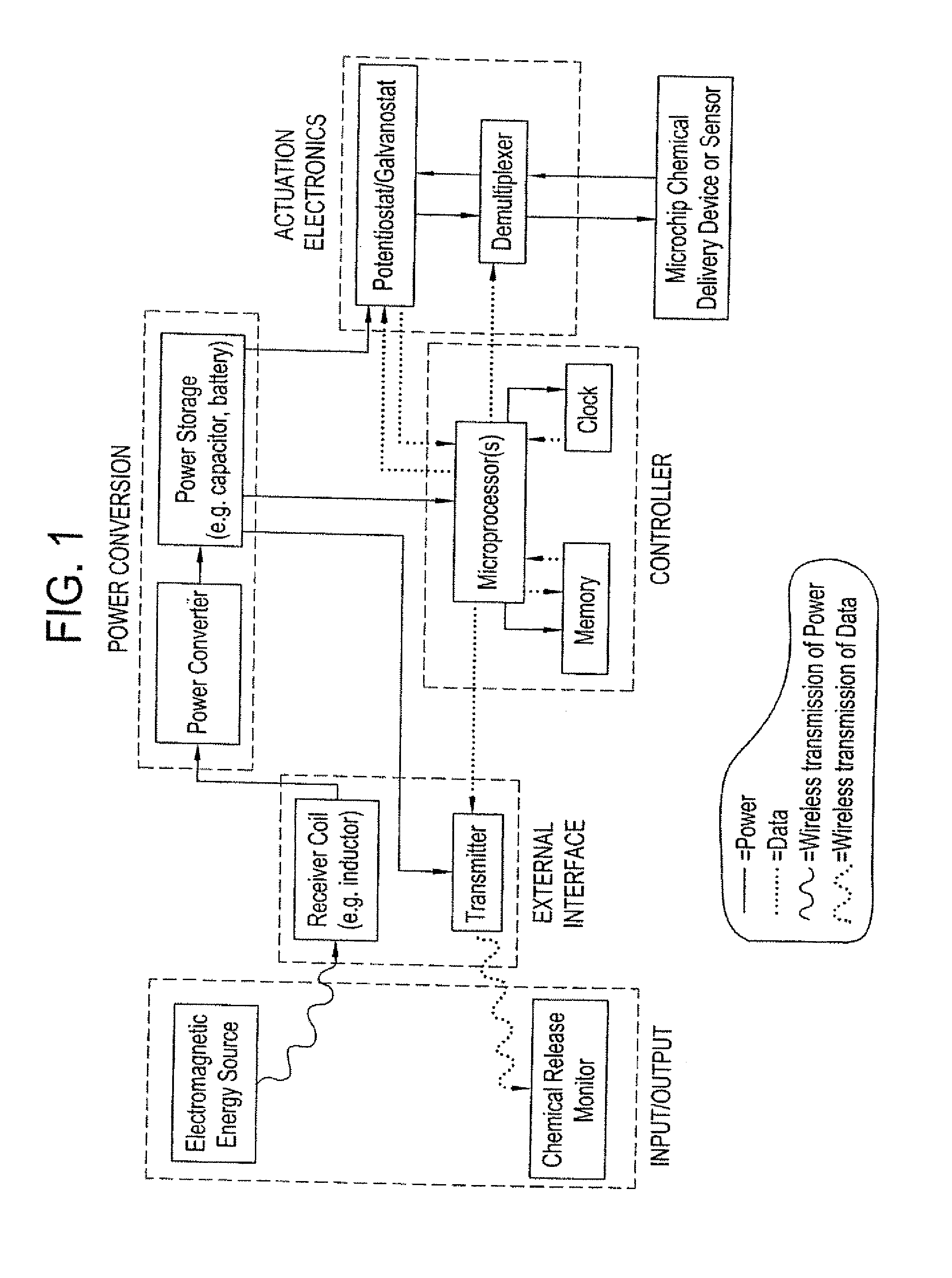
[0061] An electrochemically actuated microchip device having reservoirs covered by thin film gold reservoir caps can be fabricated using the processes described in U.S. Pat. No. 6,123,861. The reservoirs contain drug or other molecules for release. Application of an electric potential of approximately 1.0 volt (with respect to a saturated calomel reference electrode) in the presence of chloride ions would cause the reservoir caps to oxide and disintegrate, releasing the material stored in the reservoir. An on-demand power and control system would include an RF generating and a transmission source in an external controller unit. The microchip device would include a receiver coil (i.e. an inductor), an optional power converter (rectifier, regulator), a power storage unit (e.g., a capacitor, micro-battery), optional potentiostat or galvanostat circuitry (if electric potential or current modulation is ...
example 2
Microchip for the Selective Exposure of Reservoir Contents with Thermal Activation and RF Power Transmission
[0065] A microchip device similar to that described in Example 1 can be made with reservoirs containing catalysts for reactions and / or sensors for chemical and biological agent detection, wherein power transmitted by wireless methods can be used to open the reservoirs to selectively expose the contents. In this embodiment, electromagnetic energy is transmitted to a receiver coil located on or connected to the microchip device. The induced AC current can be rectified to DC current to charge a storage battery or capacitor, or sent directly to resistors located on, near, or inside the reservoirs that are to be opened. Current passing through the resistor will cause a temperature rise in the resistor and surrounding area. The rise in temperature can cause the reservoir cap material to disintegrate, melt, or phase change and selectively expose the sensor or catalyst. Alternatively...
example 3
Microchip for Release of Drug to the Eye Using Laser Actuation
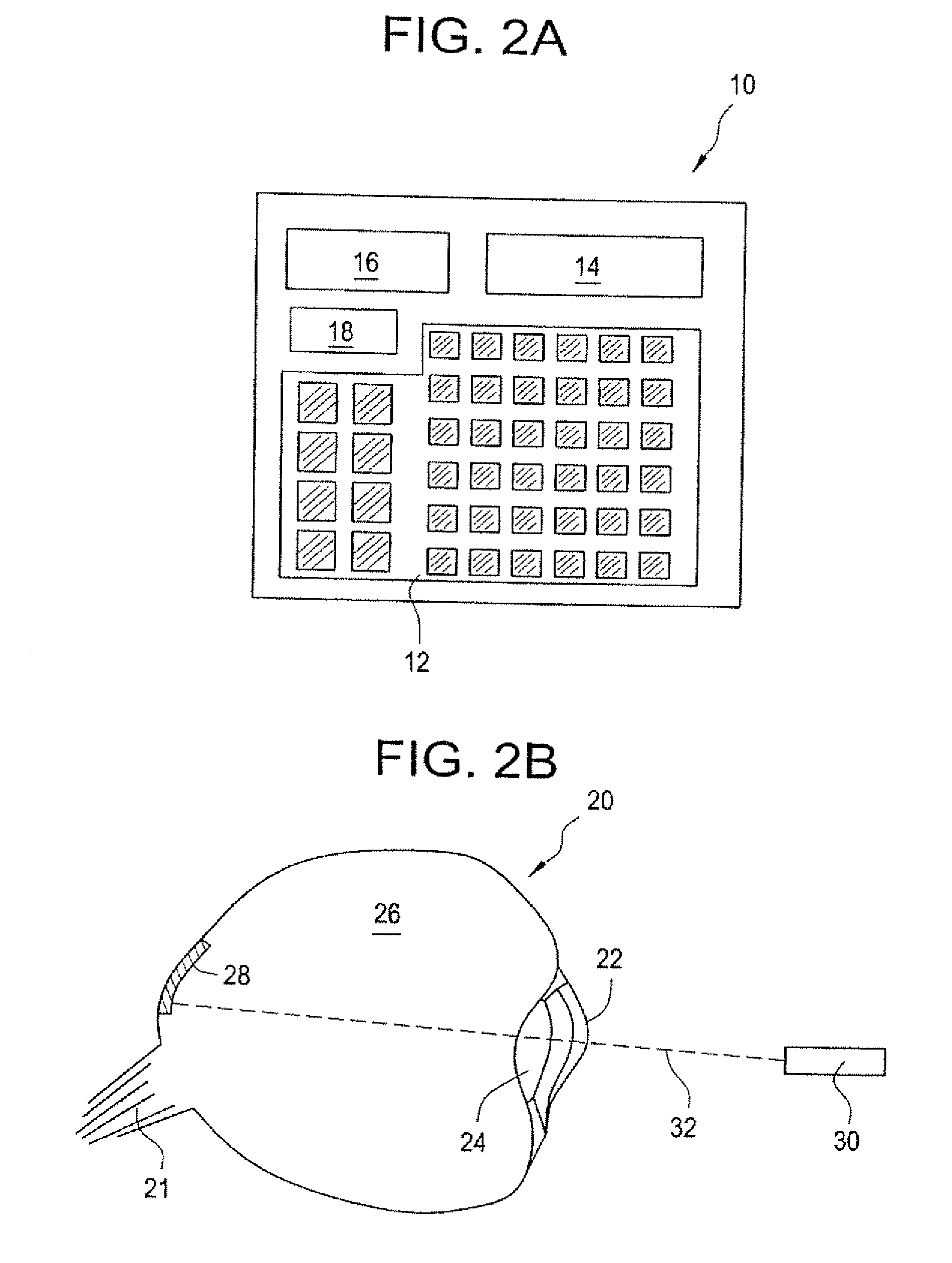
[0066] Lasers are used routinely in eye surgeries and other eye procedures for the treatment of conditions such as diabetic retinopathy, retinal detachments, age-related macular degeneration. Some conditions, notably macular degeneration, can be treated with periodic administration of medication delivered to the eye; however currently available means for doing so, such as injections, are difficult. To overcome such difficulties, an implantable microchip device can be provided to deliver doses of one or more types of medication to the eye on a periodic basis for an extended period of time. As the power requirements for electrochemically actuated silicon microchip devices with thin film gold reservoir caps are sufficiently small, the power can be wirelessly supplied, for example, via an ophthalmic laser. The ophthalmic laser also could be used to wirelessly communicate instructions to the implanted microchip device. Both p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com