Liquid Preparation of Physiologically Active Peptide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Preparations Containing Human Ghrelin (2)
[0107]The following buffers were prepared as described below: 0.5M sodium acetate buffer, 0.5M ammonium acetate buffer, 0.5M acetic acid buffer, 0.5M sodium phosphate buffer, 0.5M citric acid buffer, 0.5M glycine hydrochloride buffer, 1M lactic acid buffer, 0.06M propionic acid buffer and 0.06M n-butyric acid buffer. The pH of each buffer is shown in the parenthesis.
(1) 0.5M Sodium Acetate Buffer (pH 7.0)
[0108]Purified water was added to 8.20 g sodium acetate (MW=82.03) to a volume of 100 mL. To this solution, 1M hydrochloric acid was added to a pH of 7.0. Purified water was further added to a final volume of 200 mL.
(2) 0.5M Sodium Acetate Buffer (pH 4.0)
[0109]Purified water was added to 8.20 g sodium acetate (MW=82.03) to a volume of 100 mL. To this solution, 1M hydrochloric acid was added to a pH of 4.0. Purified water was further added to a final volume of 200 mL.
(3) 0.5M Sodium Acetate Buffer (pH 5.0)
[0110]Purified water wa...
example 2
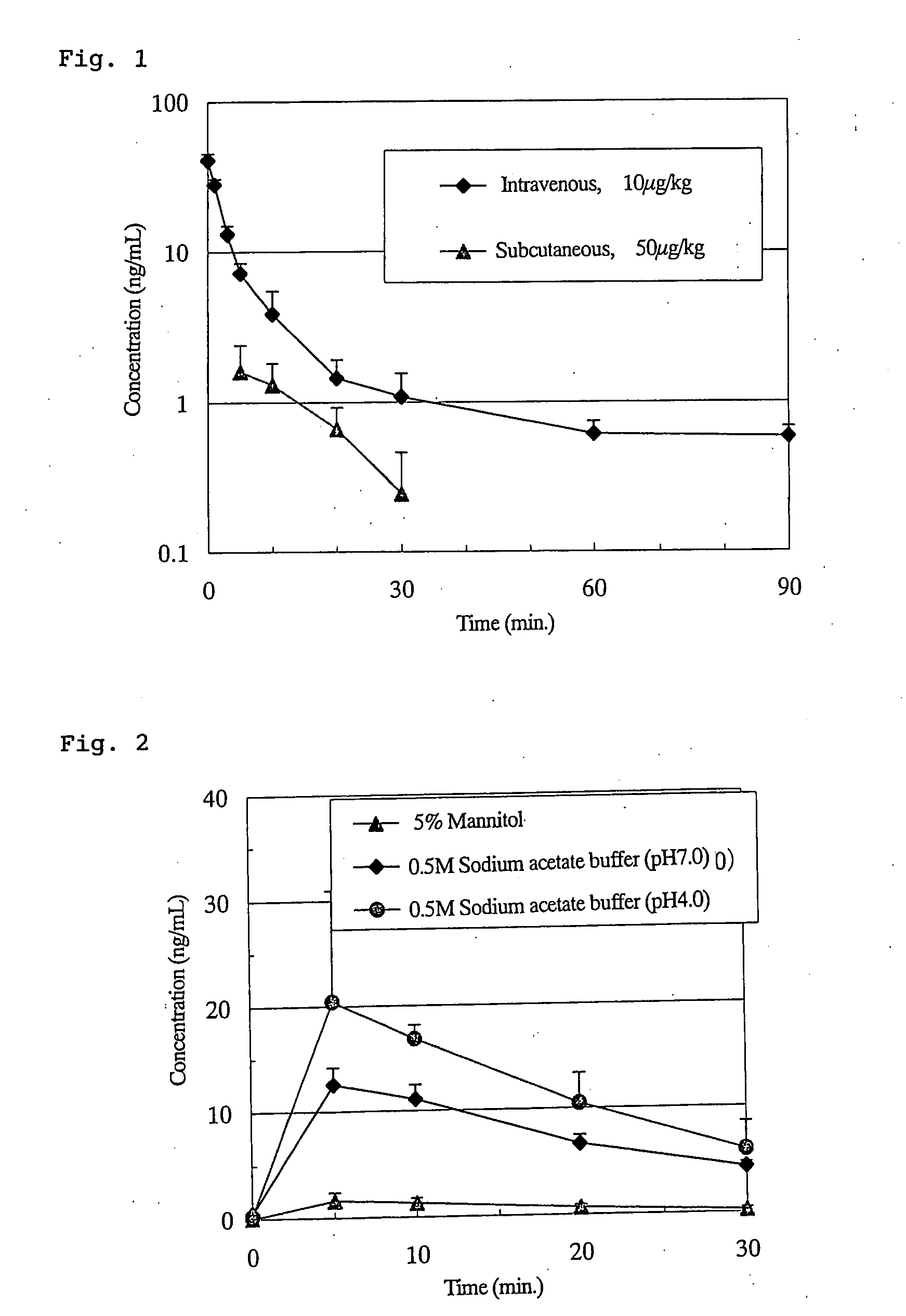
Effects of Sodium Acetate Buffers on Pharmacokinetics of Subcutaneously Injected Ghrelin in Rats
[0132]As in Comparative Example 2, Preparations No. 34, No. 6 and No. 7 were subcutaneously administered at a dose of 1 mL / kg and the plasma ghrelin levels were measured by RIA method. The results are shown in FIG. 2. The pharmacokinetic parameters are shown in Table 7 below.
Table 7: Pharmacokinetic Parameters of Ghrelin Preparations Subcutaneously Administered to Rats (Effects of Sodium Acetate Buffer)
[0133]
TABLE 7Dose volumeCmaxTmaxAUCBAPrepar. No.(mL / kg)(ng / mL)(min)(ng · min / mL)(%)341 3.35 ± 1.285.00 ± 0.00 51.94 ± 21.60 5.0 ± 2.16112.57 ± 1.625.00 ± 0.00235.38 ± 22.5922.9 ± 2.27122.29 ± 8.978.33 ± 2.89363.38 ± 16.6435.3 ± 1.6
[0134]As can be seen from the results of Table 7, the BAs obtained for Preparations No. 6 and No. 7 were 22.9% and 35.3%, respectively. These were surprisingly high as compared to the BA for Preparation No. 34 with physiological saline (5.0%) and the BA for Prepar...
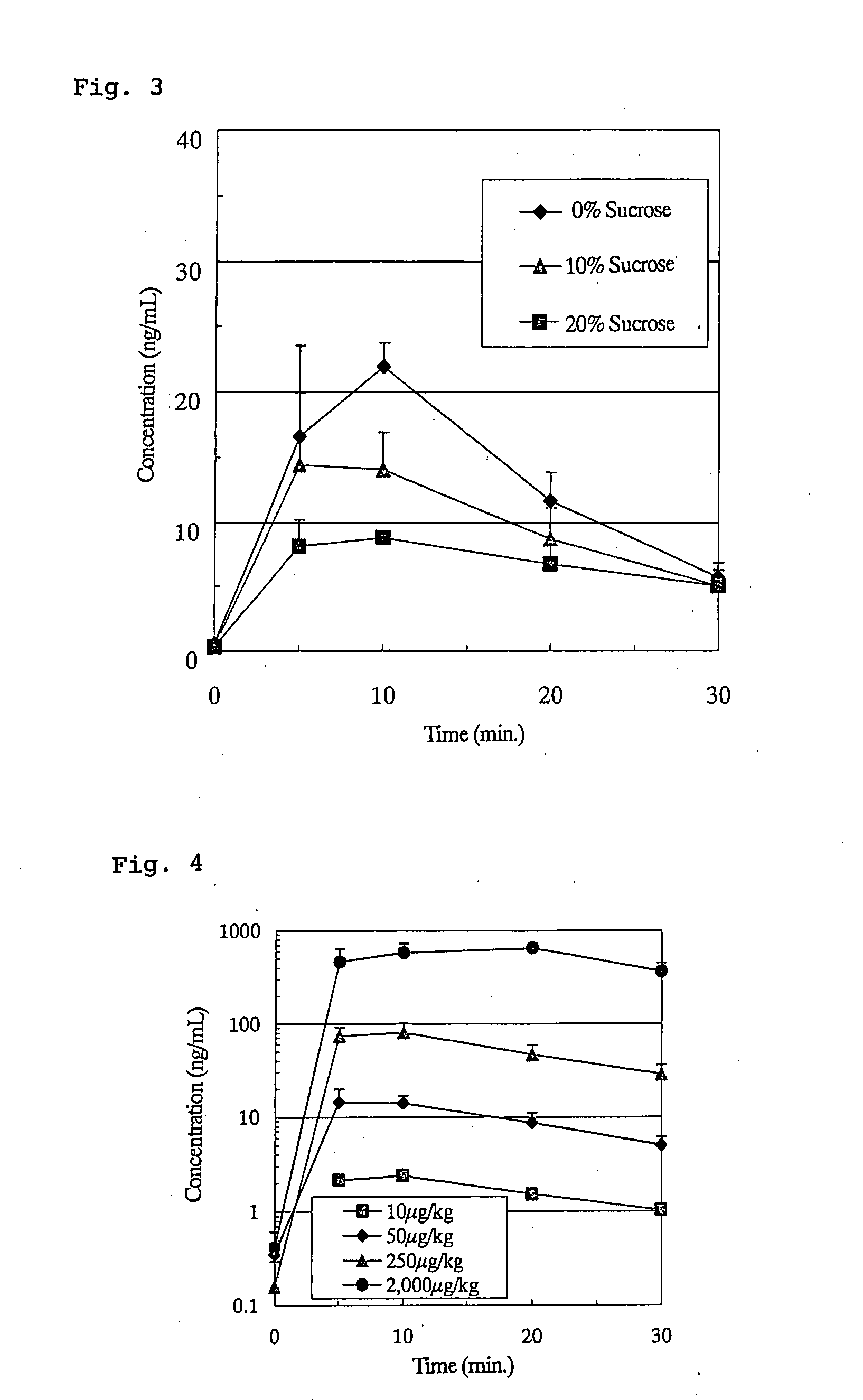
example 3
Effects of the Type of Acid Solution on Pharmacokinetics of Subcutaneously Injected Ghrelin in Rats
[0135]As in Comparative Example 2, Preparations No. 7, No. 8, No. 9, No. 10 and No. 11 were subcutaneously administered at a dose of 1 mL / kg and the plasma ghrelin levels were measured by RIA method. The pharmacokinetic parameters are shown in Table 8 below.
Table 8: Pharmacokinetic Parameters of Ghrelin Preparations Subcutaneously Administered to Rats (Effects of Types of Acid Solution)
[0136]
TABLE 8Dose volumeCmaxTmaxAUCBAPrepar. No.(mL / kg)(ng / mL)(min)(ng · min / mL)(%)7122.29 ± 8.978.33 ± 2.89363.38 ± 16.6435.3 ± 1.68113.29 ± 4.746.67 ± 2.89214.93 ± 41.0120.9 ± 4.091 3.70 ± 1.0913.33 ± 5.77 72.02 ± 10.33 7.0 ± 1.0101 6.12 ± 3.606.67 ± 2.89 91.68 ± 76.60 8.9 ± 7.411110.99 ± 5.405.00 ± 0.00167.50 ± 61.1116.3 ± 5.9
[0137]The BAs obtained for Preparations No. 7, No. 8, No. 9, No. 10 and No. 11 were 35.3%, 20.9%, 7.0%, 8.9% and 16.3%, respectively. Thus, the BA of ghrelin was markedly increa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com