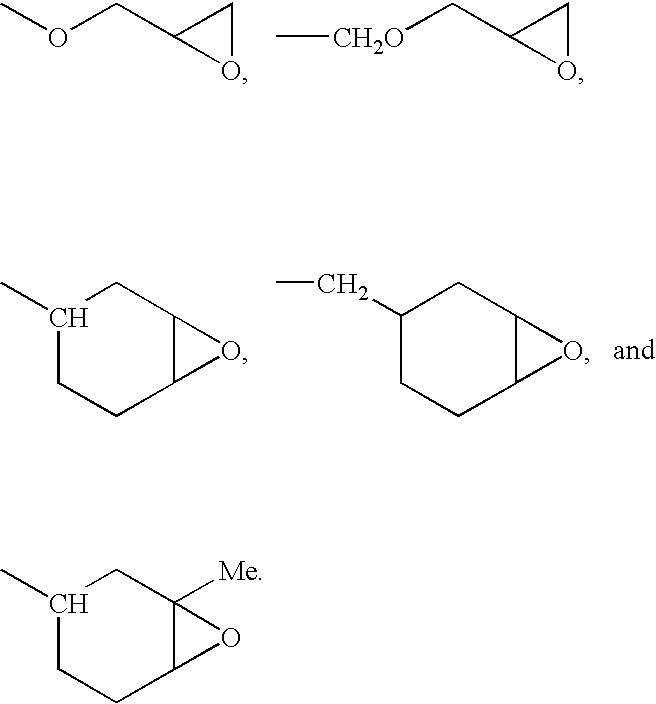
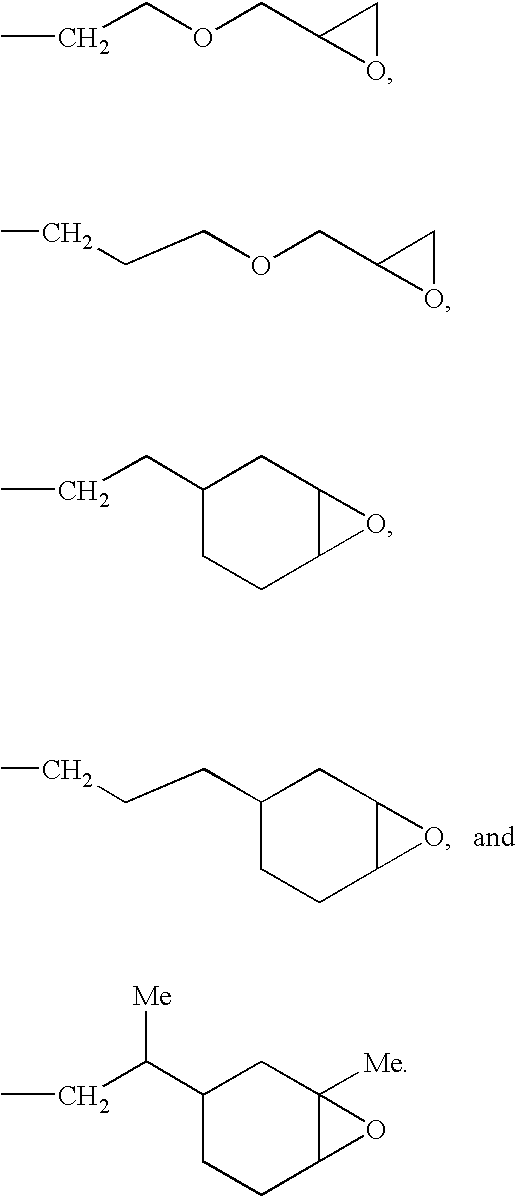
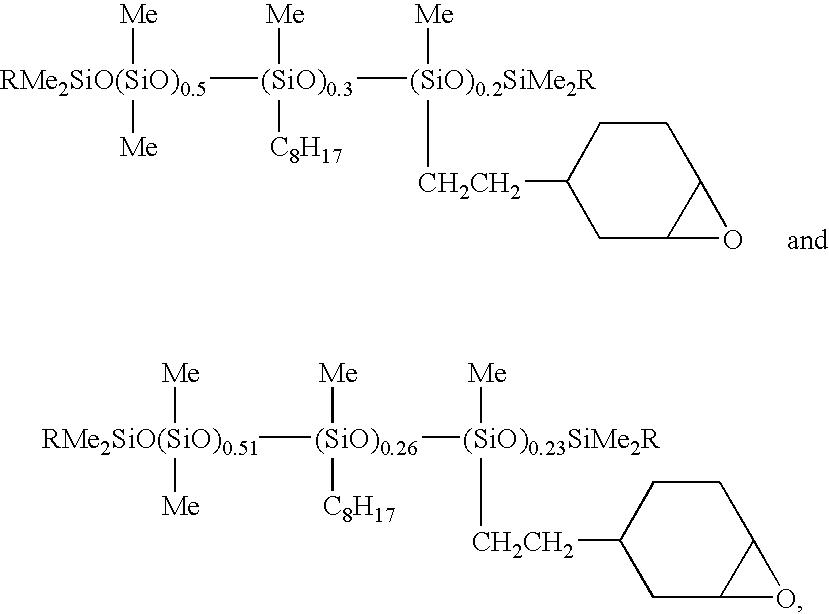
Epoxy-Functional Polysiloxanes, Silicone Composition, and Coated
a technology of polysiloxanes and polysiloxanes, applied in the field of polysiloxanes with polyfunctional properties, can solve the problems of fibers being susceptible to irreversible environmental degradation, time-consuming and expensive, and difficult to cure, and achieves good shelf-stability, low absorbance, and rapid cure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0114]Dimethylcyclosiloxanes (267.3 g, ˜3.61 mol of D4), 216.6 g (˜3.61 mol of DH4) of Methylhydrogencyclosiloxanes, and 9.97 g (0.074 mol) of 1,1,3,3-tetramethyldisiloxane were combined in a flask equipped with a condenser, agitator, and heating mantle. The mixture was treated with 5 g of Tonsil® Optimum clay catalyst. The mixture was heated to 65° C. with agitation and kept at this temperature for 2 h. The mixture was then heated to 80° C. and kept at this temperature until equilibrium was reached, as indicated by a constant ratio of cyclosiloxanes to linear polysiloxane in the 29Si NMR spectrum of the mixture. The mixture was allowed to cool to room temperature and then filtered through Grade 4 Whatman® filter paper to remove the catalyst. The filtrate was stripped (distilled) at 130° C. and 0.1 mm Hg (13.3 Pa) to remove most of the cyclosiloxanes. The residue consisted of a dimethylhydrogensiloxy-terminated poly[dimethylsiloxane-co-(methylhydrogensiloxane)] having the formula HM...
example 2
[0115]1-Octene (43.33 g, 0.39 mol) and 47.94 g (0.39 mol) of 4-vinyl-1-cyclohexene 1,2-epoxide were combined in a flask equipped with an agitator, thermometer, and addition funnel. The mixture was heated to 70° C. and 3.83 g of platinum (0.5%) on alumina was added to the flask. The dimethylhydrogensiloxy-terminated poly[dimethylsiloxane-co-(methylhydrogensiloxane)] of Example 1 (100 g, 0.77 mol silicon-bonded hydrogen atoms) was added to the mixture at such a rate as to keep the temperature below 100° C. The progress of the reaction was monitored by periodically withdrawing an aliquot of the mixture for FT-IR analysis. When the Si—H absorption at 2100-2200 cm−1 was no longer evident, the mixture was allowed to cool to room temperature. The mixture was filtered through a membrane (1 μm) to remove the catalyst, and the filtrate was passed through a Pope Wiped-Film Still at a temperature of 120° C. and pressure of 0.05 mm Hg (6.7 Pa) to give an epoxy-functional polysiloxane having the ...
example 3
[0116]An epoxy-functional polysiloxane having the formula:
where R is —C8H17 or
m is 54, n is 36, and p is 15, as determined by 29Si NMR, was prepared according to the method of Example 2, except 69.81 g (0.62 mol) of 1-octene and 23.41 g (0.1885 mol) of 4-vinyl-1-cyclohexene 1,2-epoxide were used in the reaction. In the preceding formula the subscripts m, n, and p denote mole fractions, which are based on the total number of moles of repeat (non-terminal) siloxane units in the polysiloxane. The polysiloxane was obtained as an colorless fluid having a refractive index, η20D, of 1.4689 and a viscosity of 578 cS (578 mm2 / s).
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelengths | aaaaa | aaaaa |
| wavelengths | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com