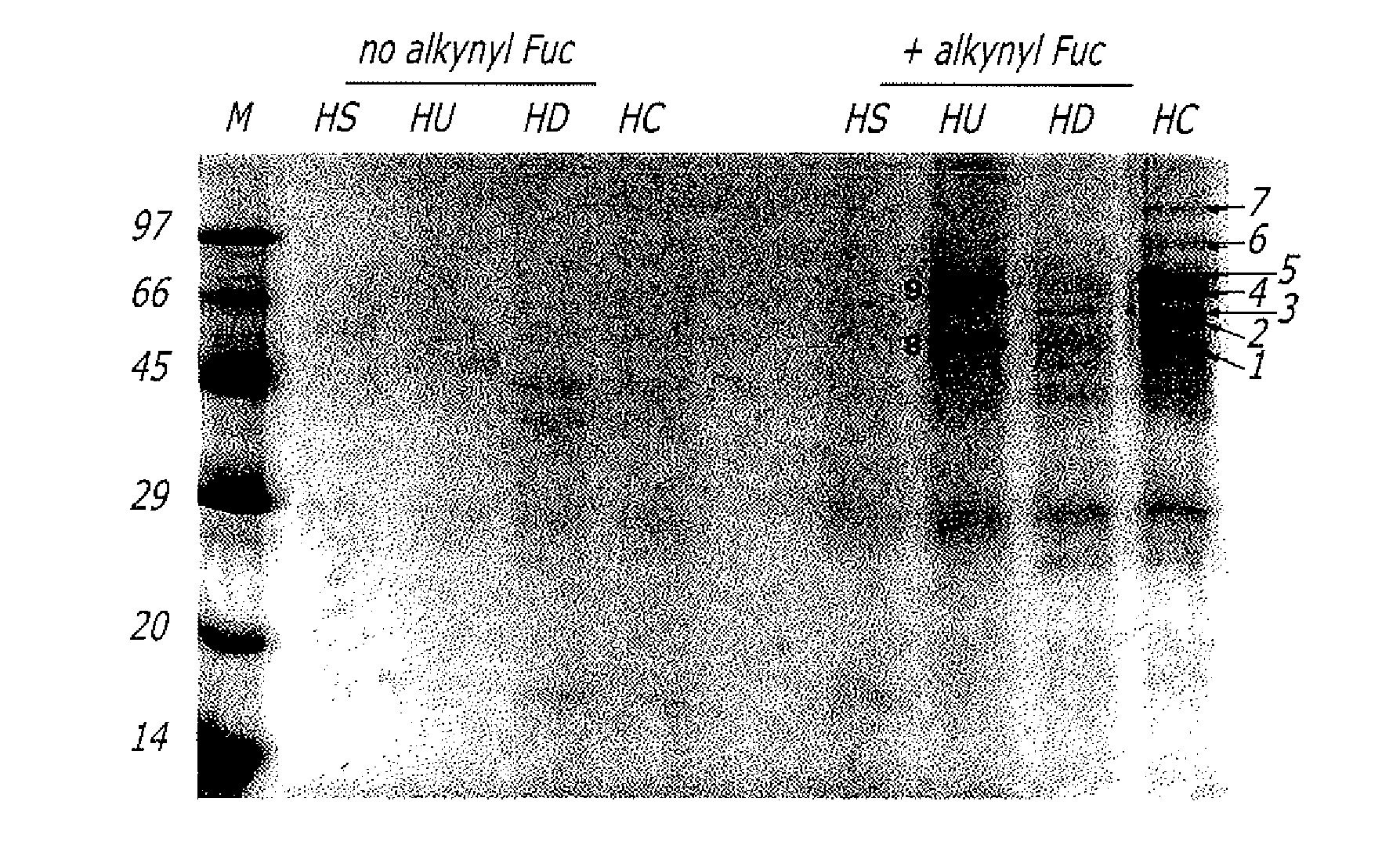
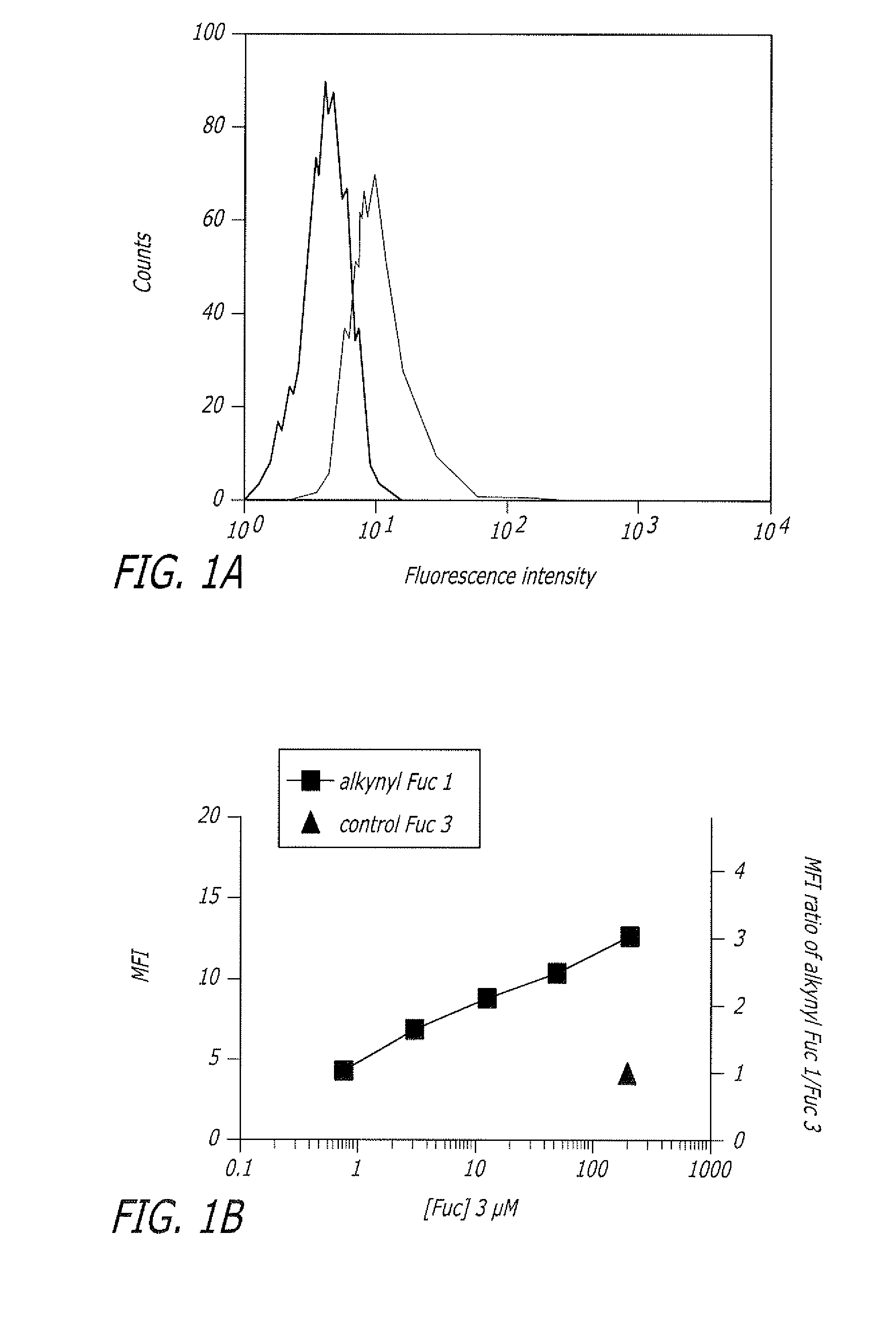
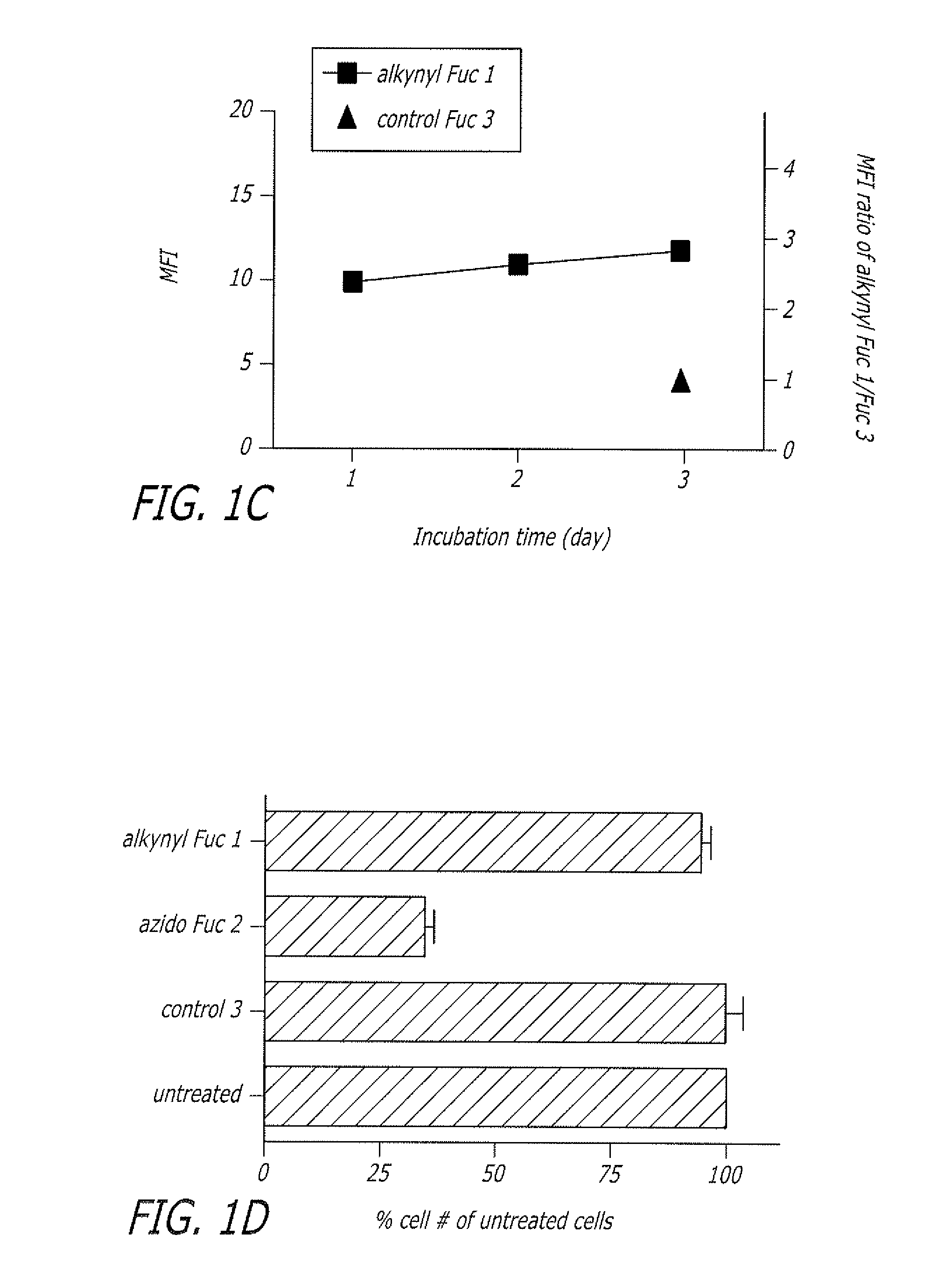
Alkynyl sugar analogs for the labeling and visualization of glycoconjugates in cells
a glycoconjugate and alkyl sugar analog technology, applied in the field of metabolic oligosaccharide engineering, can solve the problems of complex glycan structure identification and molecular level delineation of their mode of action, complex glycan structure, and low glycan complexity, so as to improve the effect of toxicity and the improvement of the method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of 1,2,3,4-tetraacetyl alkynyl fucose (Fuc) (1, mixture of anomers; Scheme 2)
[0302]To a flask containing compound 8 (0.05 g, 0.2 mmol), TFA solution (1 ml, 90% TFA in H2O) was slowly added at 0° C. The reaction was stirred on ice for 1 h and concentrated in vacuo. The resulting residue was treated with pyridine (1 ml), N,Ndimethylaminopyridine (2.0 mg), and acetic anhydride (1 ml), stirred overnight, concentrated, and diluted with dichloromethane. This solution was then sequentially washed with 1 N aqueous HCl, saturated aqueous NaHCO3, and brine. The organic phase was dried over anhydrous Na2CO3 and concentrated. Silica gel chromatography gave product 1 (0.055 g, 80%, □-pyranoside:□-pyranoside: □-furanoside:□-furanisude=30:51:11:8) as a colorless gum (FIG. 7). Partial 1H-NMR of mixture (500 MHz, CDCl3) □ 5.74 (d, J=8.4 Hz, H-1 (□pyr)), 6.24 (s, H-1 (□fur)), 6.36 (d, J=4.8 Hz, H-1 (□fur)), 6.43 (d, J=2.6 Hz, H-1 (□pyr)); ESI-TOF-HRMS m / e calculated for (M+Na)+ C15H18O9Na 3...
example 2
Synthesis of N-4-pentynoylmannosamine (10, mixture of anomers; Scheme 3)
[0303]A mixture of D-mannosamine hydrochloride (863 mg, 4.0 mmol), N-succinimidyl 4-pentynoate 9 (781 mg, 4.0 mmol), triethylamine (1.67 ml, 12.0 mmol) in DMF (31 ml) was stirred at room temperature overnight. The reaction mixture was concentrated in vacuo, and the residue was purified by flash column chromatography (CHCl3 / MeOH 8:1) to give N-4-Pentynoylmannosamine, 10 (898 mg, 87%); 1H-NMR (500 MHz, D2O) 2.37 (t, 2.63H, J=2.5 Hz), 2.48-2.63 (m, 10.5H), 3.38-3.42 (m, 1H), 3.52 (t, 1H, J=10 Hz), 3.63 (t, 1.63H, J=10 Hz), 3.69-3.91 (m, 7.89H), 4.05 (dd, 1.63H, J=4.5 and 10 Hz), 4.35 (dd, 1.63H, J=1.5 and 4.5 Hz), 4.47 (dd, 1H, J=1.5 and 4.5 Hz), 5.03 (d, 1H, J=1.5 Hz), 5.13 (d, 1.63H, J=1.5 Hz); 13C-NMR (125 MHz, D2O)□ 14.78, 14.91, 34.62, 34.79, 53.67, 54.50, 60.91, 60.93, 67.01, 67.28, 69.25, 70.56, 70.71, 72.47, 72.50, 76.80, 84.04, 84.45, 93.36, 93.67, 175.68, 176.41; ESI-TOF-HRMS m / e calculated for (M+H)+ C1...
example 3
Synthesis of 1,3,4,6-tetra-O-acetyl-N-4-pentynoylmannosamine (4, mixture of anomers; Scheme 3)
[0304]A mixture of 10 (123 mg, 0.500 mmol) and acetic anhydride (0.227 ml, 2.40 mmol) in pyridine (4 ml) was stirred at room temperature overnight. The reaction mixture was concentrated in vacuo, and the residue was dissolved in CH2Cl2 and washed with water. The organic layer was dried over Na2SO4 and evaporated. The residue was purified by flash column chromatography (AcOEt / Hexane 1:4) to give 1,3,4,6-tetra-O-acetyl-N-4-pentynoylmannosamine, 4 (183 mg, 86%); 1H-NMR (500 MHz, CDCl3) □ 2.00 (s, 9H), 2.06 (s, 9H), 2.097 (s, 3H), 2.10 (s, 3H), 2.11 (s, 3H), 2.14-2.18 (m, 3H), 2.19 (s, 6H), 2.46-2.58 (m, 12H), 3.81-3.87 (m, 1H), 4.00-4.15 (m, 5H), 4.23-4.30 (m, 3H), 4.69 (dd, 2H, J=4.5 and 10 Hz), 4.82 (dd, 1H, J=4.5 and 10 Hz), 5.09 (dd, 1H, J=4.5 and 10 Hz), 5.17 (t, 1H, J=10 Hz), 5.23 (t, 2H, J=10 Hz), 5.33 (dd, 2H, J=4.5 and 10 Hz), 5.90 (s, 1H), 6.03 (s, 2H), 6.36 (d, 1H, J=9.5 Hz), 6.54 (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com