Methods and compositions for nerve regeneration
a nerve regeneration and composition technology, applied in the field of methods and compositions for nerve regeneration, can solve the problems of limited treatment options for patients with spinal cord injuries, permanent impairment of neural function, damage to the spinal cord,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
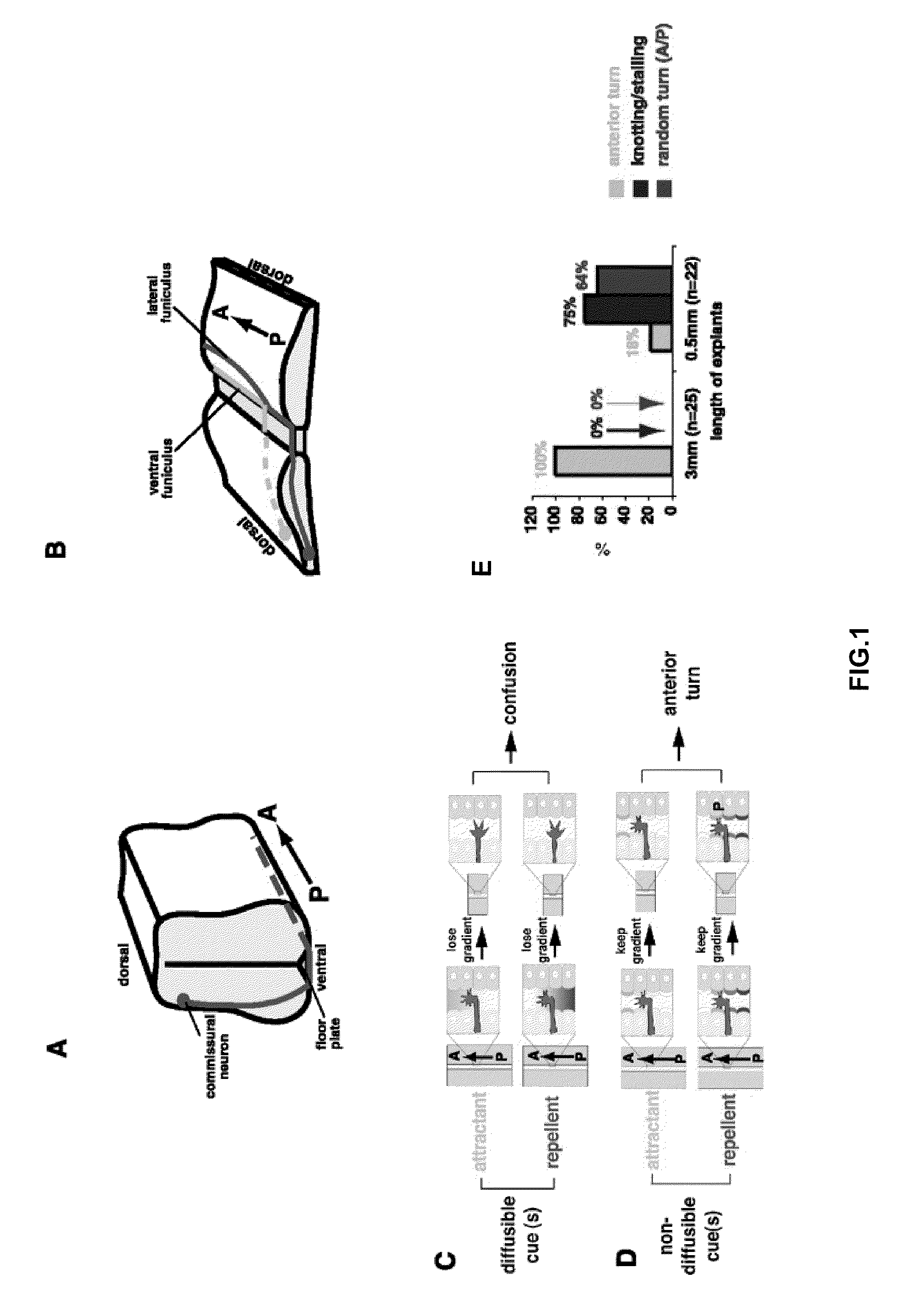
[0214]Collagen gel assays. E13 rat spinal cord explants were cultured in collagen gel matrix as described previously (Tessier-Lavigne et al., 1988; Zou et al., 2000). These explants are either “open-book” or post-crossing or pre-crossing for the spinal cord commissural axons. COS7 cells were transfected with various expression constructs with FuGene6 reagent (Roche). The explants were typically cultured for 16-20 hours and fixed in 4% PFA for two hours. The “open-book” explants were analyzed by lipophilic DiI labelling using iontophoresis. The post-crossing explants were stained with a monoclonal antibody (E7) against P3 tubulin (Hybridoma Bank for Developmental Studies). The pre-crossing explants were stained with a monoclonal antibody (4D7) against TAG-1 (Hybridoma Bank for Developmental Studies). Both antibodies were detected using secondary antibodies conjugated with horseradish peroxidase and visualized with 3,3′-diaminobenzene (DAB) (Sigma). Quantification...
example 2
The A-P Guidance Cue(s) is Diffusible
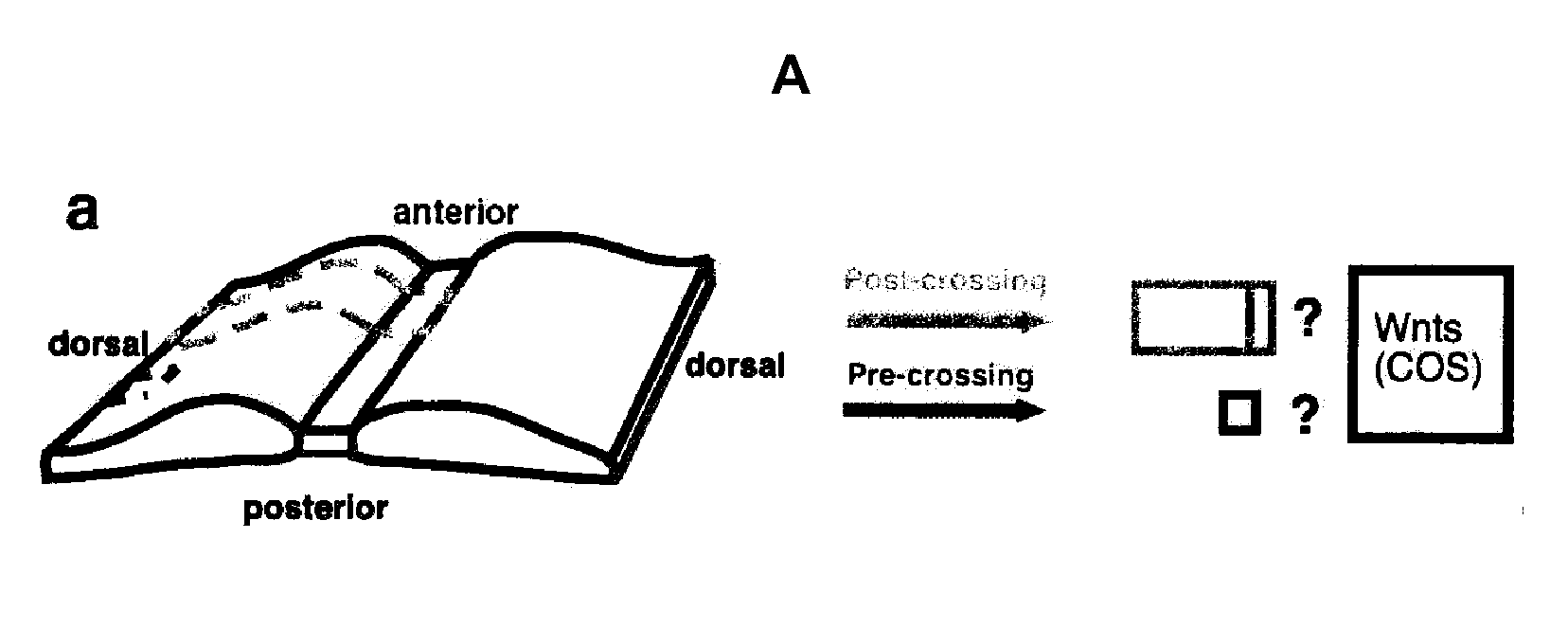
[0221]When a segment of E13 rat spinal cord is cultured in collagen gel for 16-18 hours, commissural axons were observed to project ventrally, cross the midline and turn anteriorly within the explant, mimicking their in vivo pathfinding. Commissural axon trajectories in these “open-book” explants can be revealed by lipophilic D11 injection into the dorsal side of the explants by iontophoresis (Fraser, 1996). Most of the commissural axons in E13 rat spinal cord “open-book” preparations fixed immediately after dissection (without culturing) are only just approaching the midline or in the process of midline crossing. Therefore, the midline crossing and anterior turning of the commissural axons observed with DiI labeling occurred during the “open-book” culture period.
[0222]FIG. 1A schematically demonstrates that during embryonic development, commissural neurons project axons to the ventral midline. Once they reach the floor plate, they cross the midl...
example 3
The A-P Guidance Cue(s) is Attractive
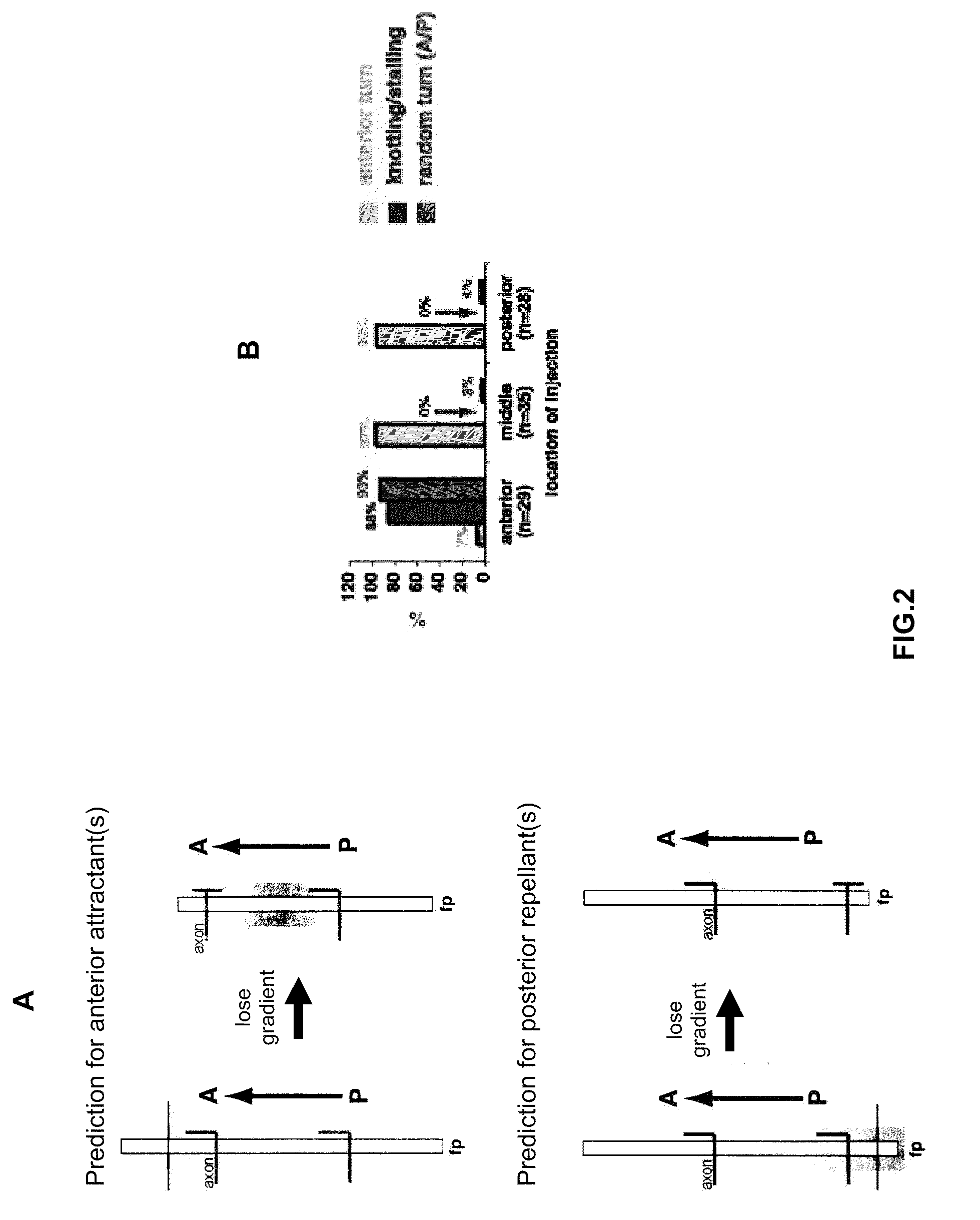
[0224]To address whether the A-P guidance cue is attractive or repulsive, DiI was focally injected into the dorsal spinal cord close to the anterior end, in the middle and close to the posterior end of the long explants (3 mm or longer). The axons in the middle and close to the posterior end of the explants were found to always project anteriorly, whereas the axons close to the anterior end almost always make mistakes: they either stall after they cross the midline or they project both anteriorly and posteriorly after midline crossing, or sometimes only posteriorly. The results were quantified using the same criteria as shown in FIG. 1E. The quantification is shown in FIG. 2B. The axons close to the anterior end of the explants behave similarly to those in the short explants (0.5 mm), whereas the axons in the middle and posterior part of the explants behave normally. These results are consistent with the possibility that a gradient of an attracti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com