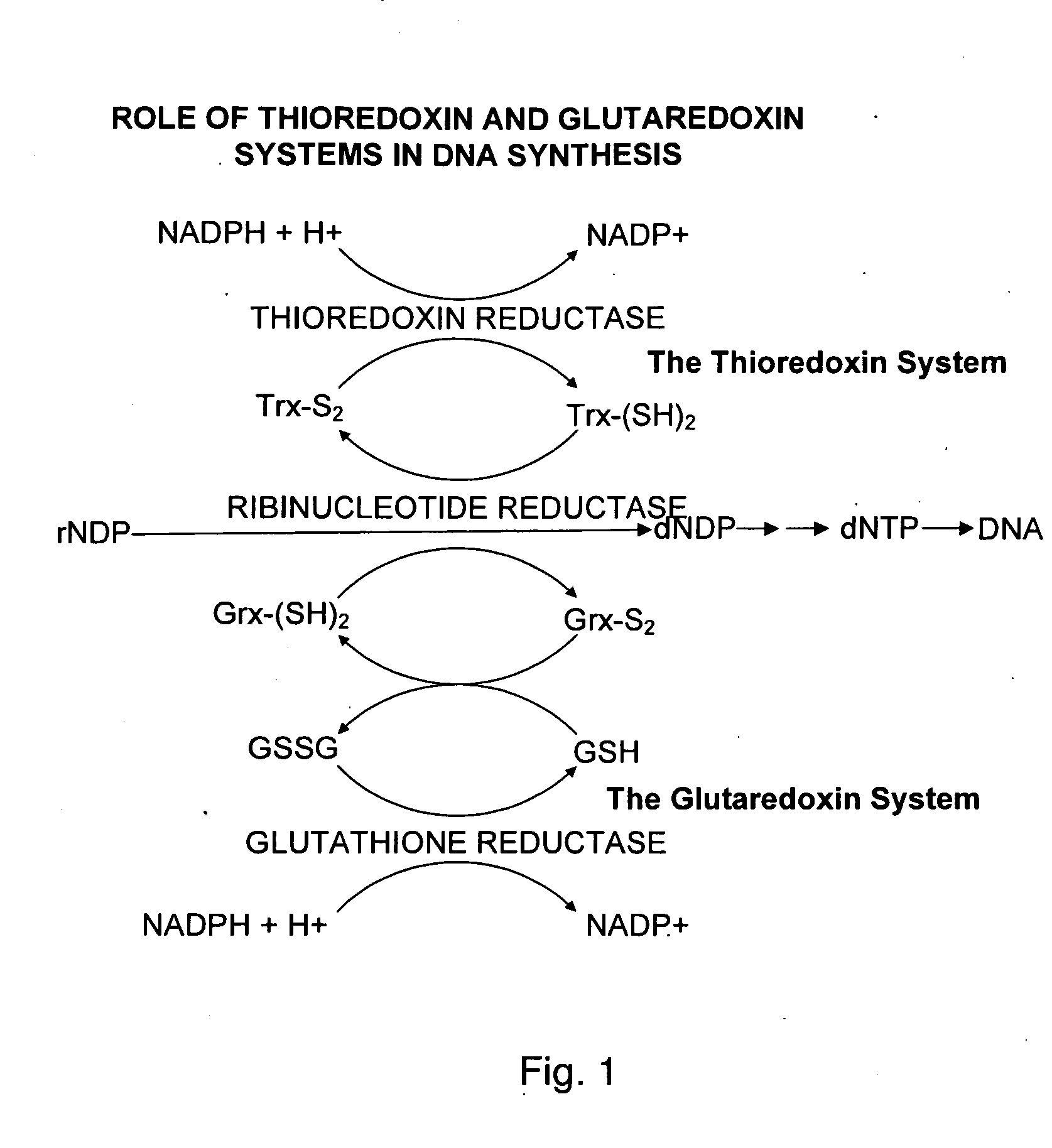
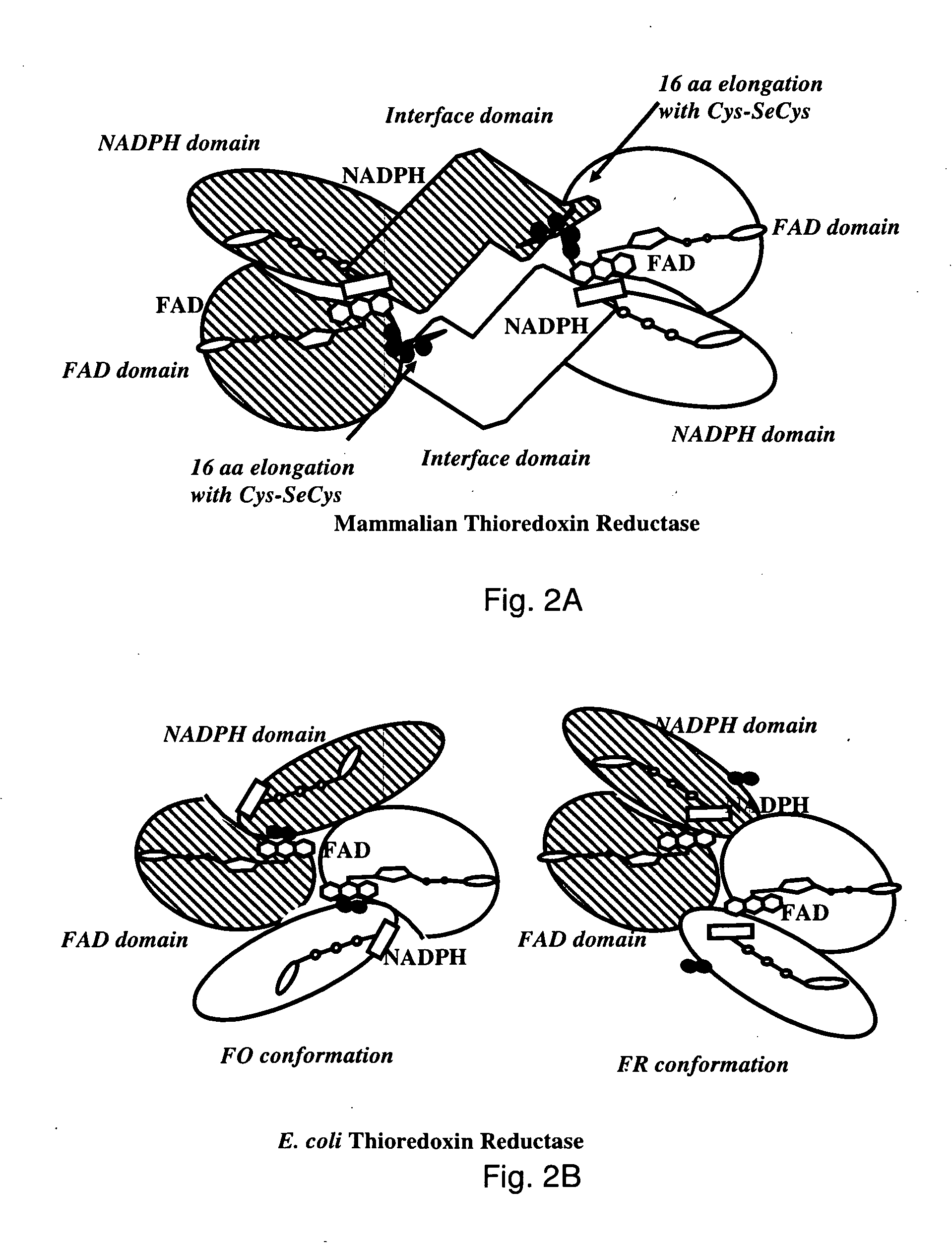
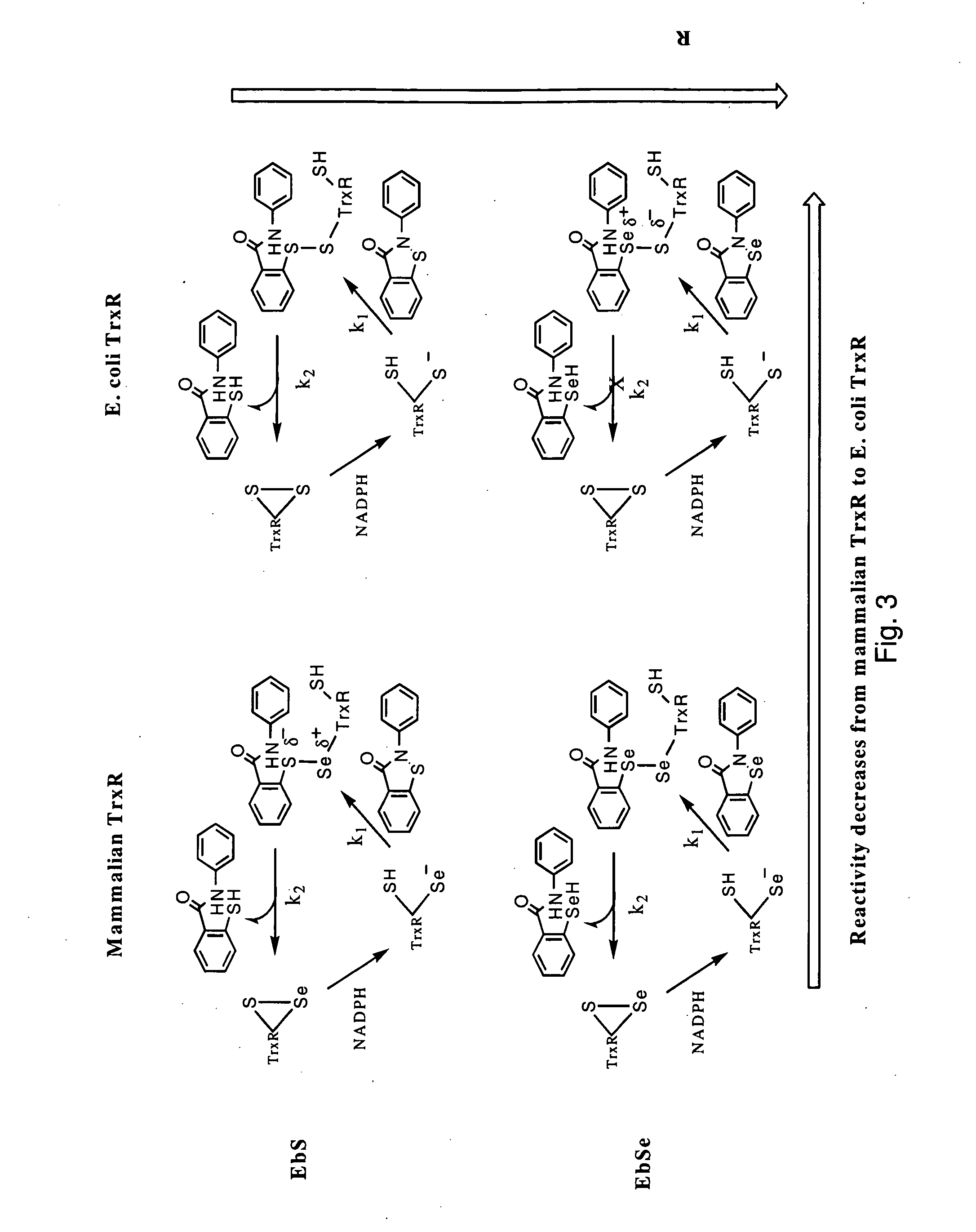
Bacterial thioredoxin reductase inhibitors and methods for use thereof
a technology of thioredoxin and reductase inhibitor, which is applied in the field of biologically active selenium and sulfur compounds, can solve the problems of increasing resistance to bacterial infections and limitations of current drugs, and achieve the effect of reducing thioredoxin and rapidly oxidizing reducing thioredoxin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0034]Experiments—Materials and Enzymes
[0035]NADPH, DTT, DTNB, DMSO, insulin, and bovine serum albumin (BSA) were acquired from Sigma-Aldrich. Calf thymus TrxR and E. coli TrxR and Trx were acquired from IMCO corporation Ltd, Stockholm, Sweden (www.imcocorp.se). Rat glutathione reductase was a pure preparation prepared according to the method previously published (4). H. pylori TrxR and Trx were prepared as described before (66). Ebselen, 14C-labelled ebselen, ebselen diselenide and PZ25 (ebsulfur) were products of Daiichi, Tokyo, Japan and were dissolved in fresh DMSO before addition into the solution. Concentrations of DMSO were less than 5% of the solvent buffer, effective in dissolving the drugs. E. coli DHB4 strain wt, gor−, gshA− were described as the reference (Prinz, W. A., Aslund, F., Holmgren, A. & Beckwith, J. (1997) J Biol Chem 272, 15661-7.)
[0036]Compounds Synthesis
[0037]All reactions were performed under inert atmosphere using Schlenk techniques. All solvents were puri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com