Steel wire material for spring and its producing method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0173]The example in relation with the first embodiment is described hereunder.
[0174]The steel with the chemical composition shown in Table 1 (steel kind: SA-SL) was molten by 80 ton converter, a 400 mm square bloom was made by continuous casting, and then it was bloomed to a 155 mm square billet. After the billet was heated it was hot-rolled, then, after water-cooled nearly to the placing temperature, it was coiled and placed onto a cooling bed (conveyor) of a Stelmor cooling device, and by being subjected to air-blast cooling with the air volume supplied to the close parts of the coil and the coarse parts of the coil being adjusted, 2 tons of wire material for a spring with a 14.3 mm diameter was produced. The detailed production conditions are as shown in Table 2. In the table 2, cooling speed is the speed between the temperatures of 750° C. and −600° C.
[0175]Tensile strength, fracture reduction of area, depth of decarburized layer of the steel obtained were measured as described...
example 2
[0187]The example in relation with the second embodiment is described hereunder.
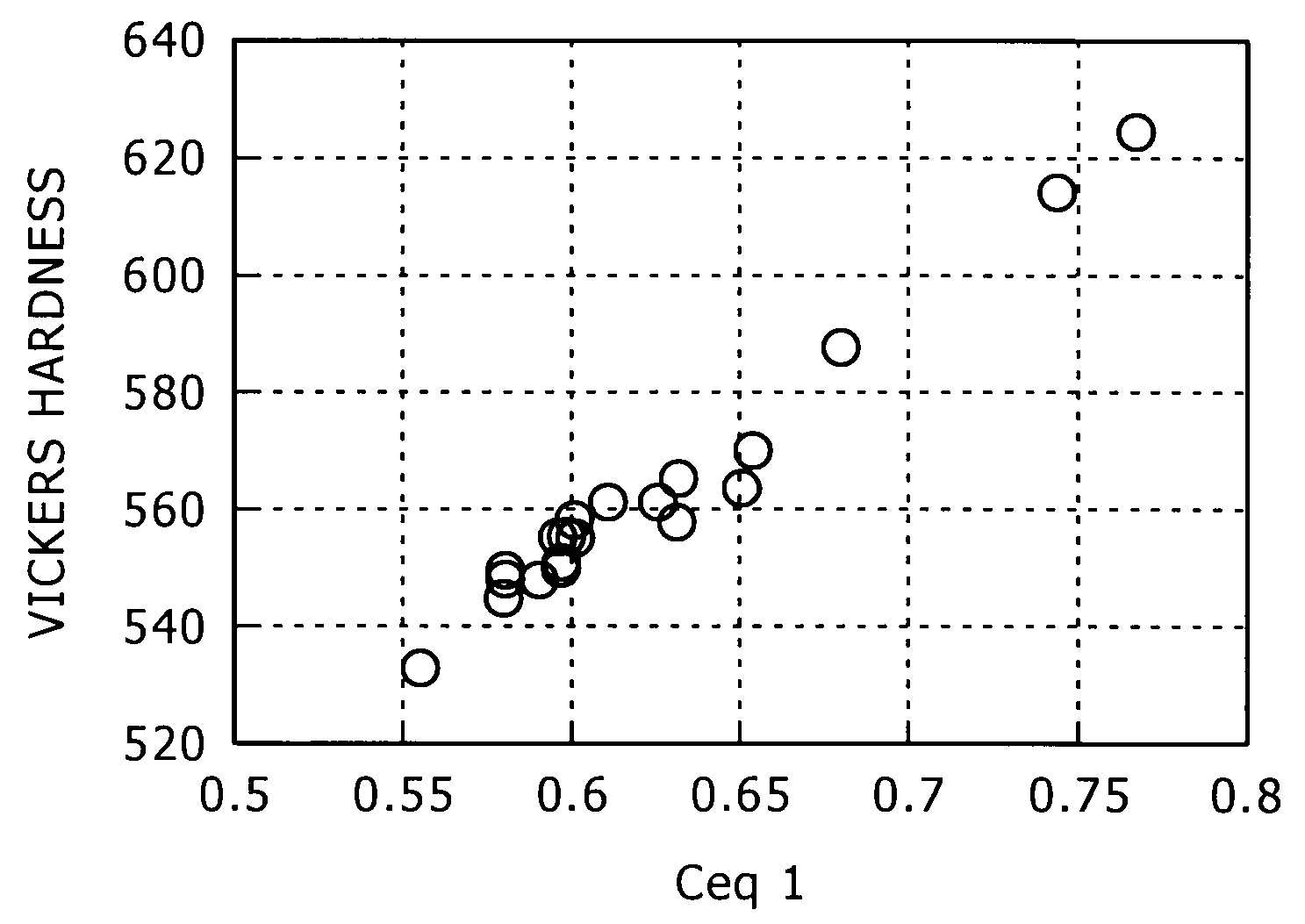
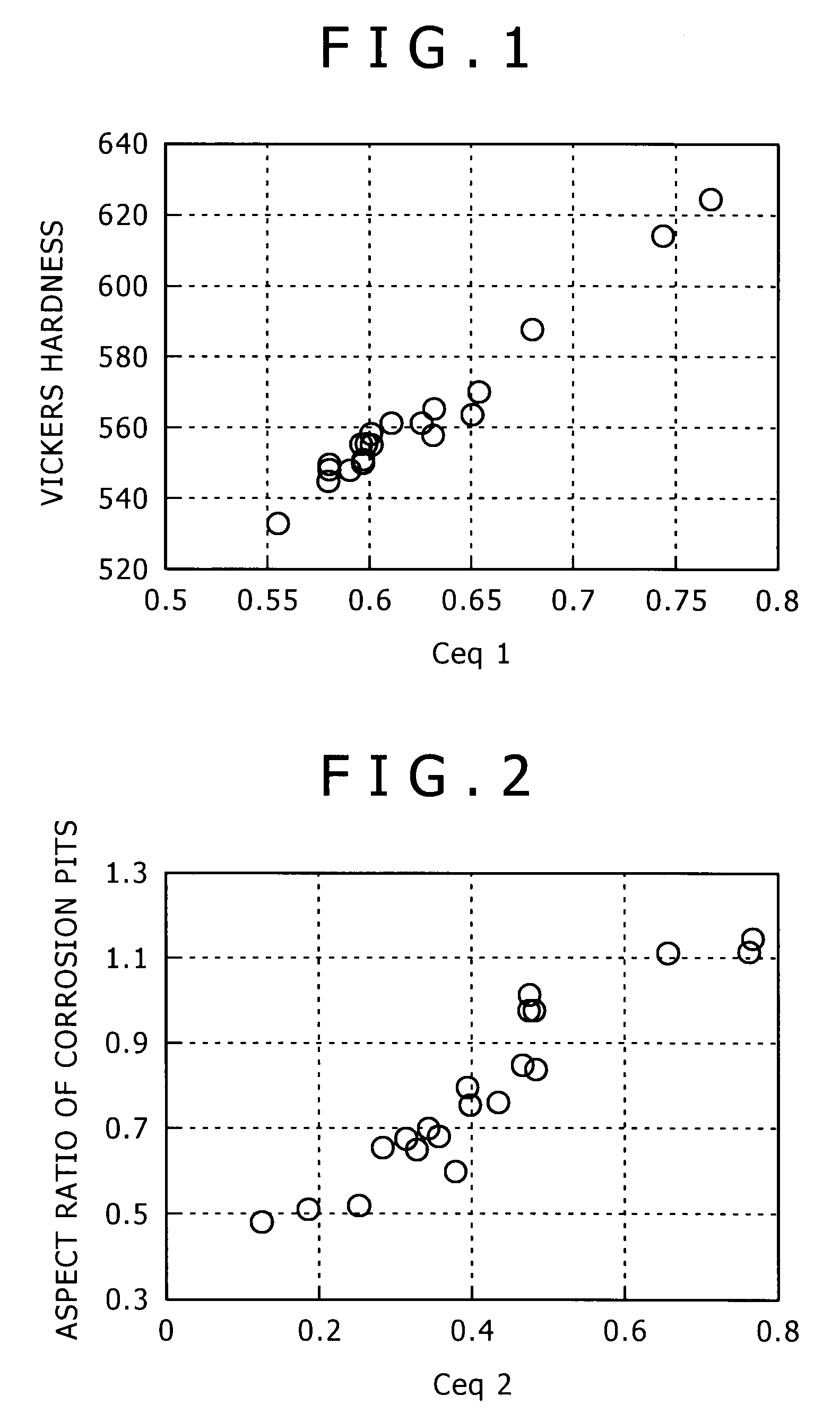
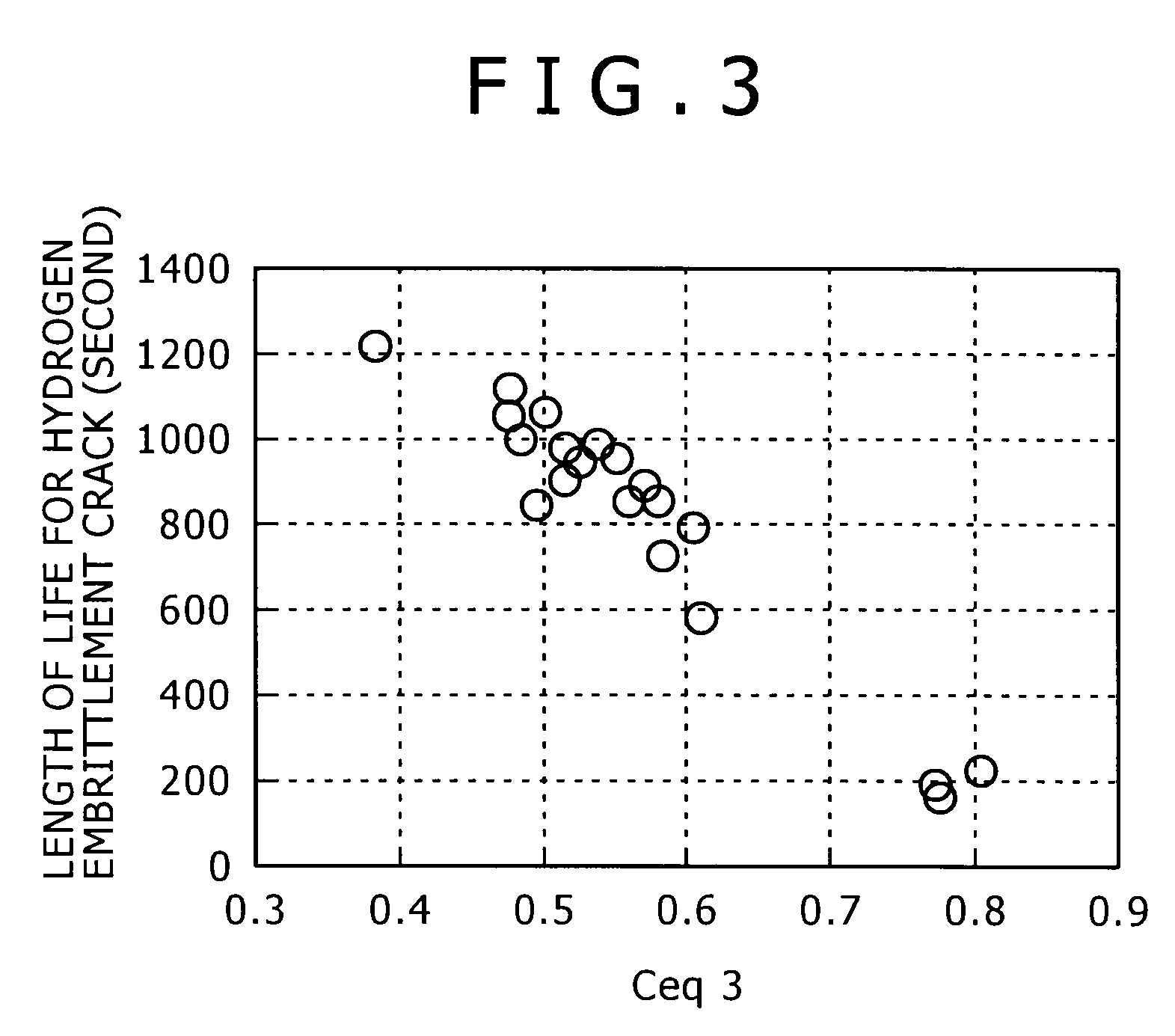
[0188]The steel with the chemical composition shown in Table 3 was molten by a 150 kg small sized vacuum melting furnace, and a 155 mm square billet was made by hot forging. Ceq1-3 calculated from the chemical composition are shown in Table 5. After the billet was heated, it was hot-rolled, then, after water-cooled nearly to the placing temperature, it was coiled and placed onto the cooling bed (conveyor) of the Stelmor cooling device, and by being subjected to air-blast cooling with the air volume supplied to the close parts of the coil and the coarse parts of the coil being adjusted, the spring steel (wire material) with a 13.5 mm diameter was produced. The detailed production conditions are as shown in Table 4. In Table 4, cooling speed is the speed between the temperature of 600° C.-750° C. Further, in Table 4, A1(c=0) transformation point, A3(c=0) transformation point, and A4(c=0) transformation poi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com