Method for producing circular or multimeric protein species in vivo or in vitro and related methods
a multimeric protein and cyclic peptide technology, applied in the field of circular or multimeric protein species production in vivo or in vitro, can solve the problems of difficult to generate synthetic cyclic peptides larger than 100 amino acids, and procedures that do not allow the cyclization of proteins or peptides in vivo for study in living organisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Creation of Vectors pMEB1, pMEB2, pMEB3 for Cis-Splicing Studies
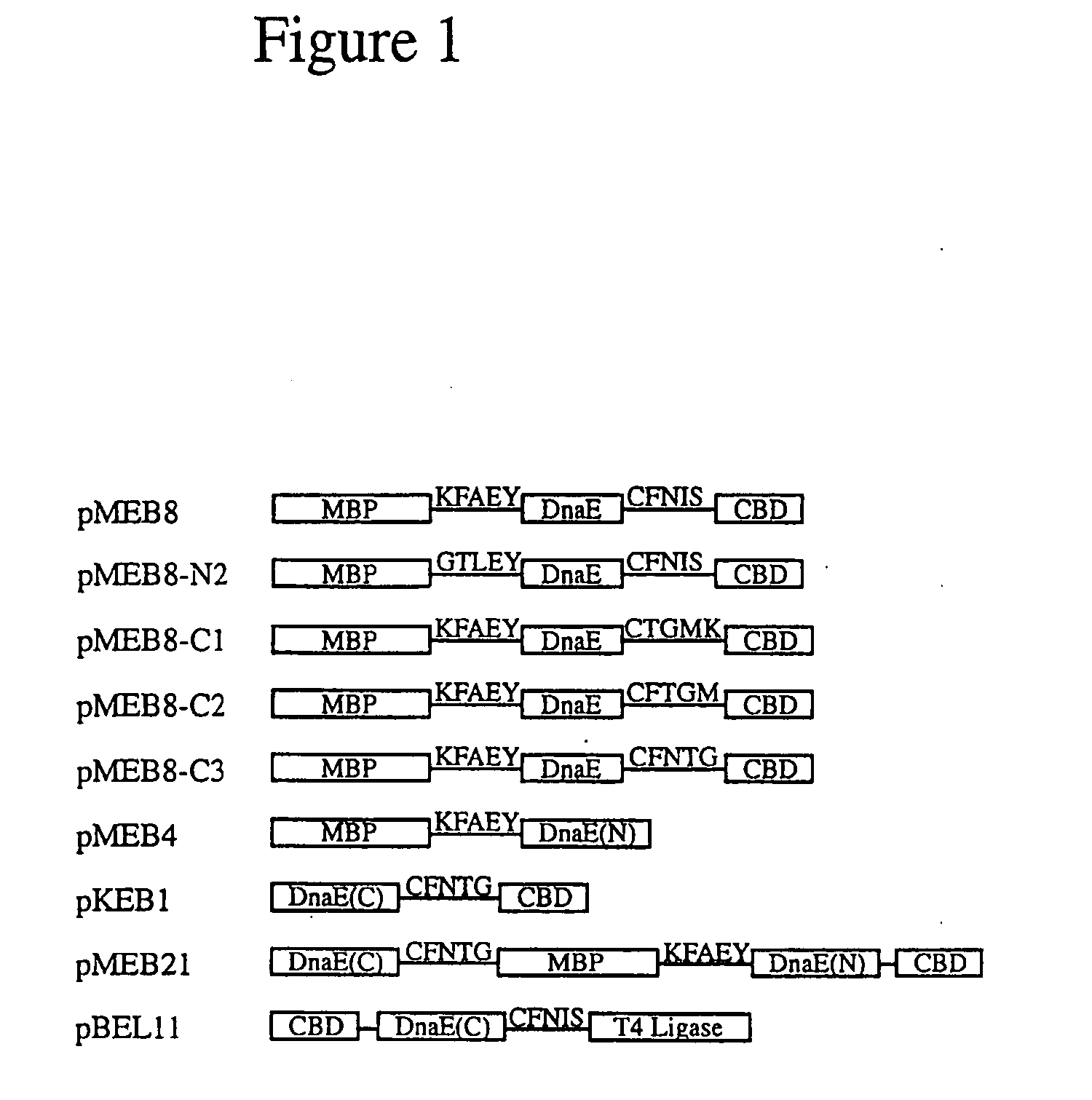
[0058]Construction of Plasmids-pMEB 1 was constructed by replacing the Sce VMA intein in pMYB129 (Chong, et al., Gene 192(2):271-281 (1997)) with the Ssp DnaE intein sequence spanning residues 5-123 to create a fusion gene composed of E. coli maltose-binding protein (MBP) (Duplay, et al., J. Biol. Chem. 259:10606-10613 (1984)), the Ssp DnaE intein (residues 5-123) and the Bacillus circulans chitin binding domain (CBD) (Watanabe, et al., J. Bacteriol. 176:4465-4472 (1994)). The Ssp DnaE intein fragment was amplified from plasmid pDnaE-C209 with primers 5′-TTTGGTACCGAAATTTTAACCGTTGAG-3′ (SEQ ID NO:1) and 5′-GGCTCTTCCTTTAATTGTCCCAGCGTCAAG-3′ (SEQ ID NO:2). The N-terminal splice junction sequence, containing the flanking 5 native N-extein residues and the 5 intein N-terminal residues, was inserted between maltose binding protein (MBP) and the intein coding regions by linker insertion into the XhoI and KpnI sites in pMEB1 to...
example ii
The Cis-Splicing Activity of the Ssp DnaE Intein In Vivo
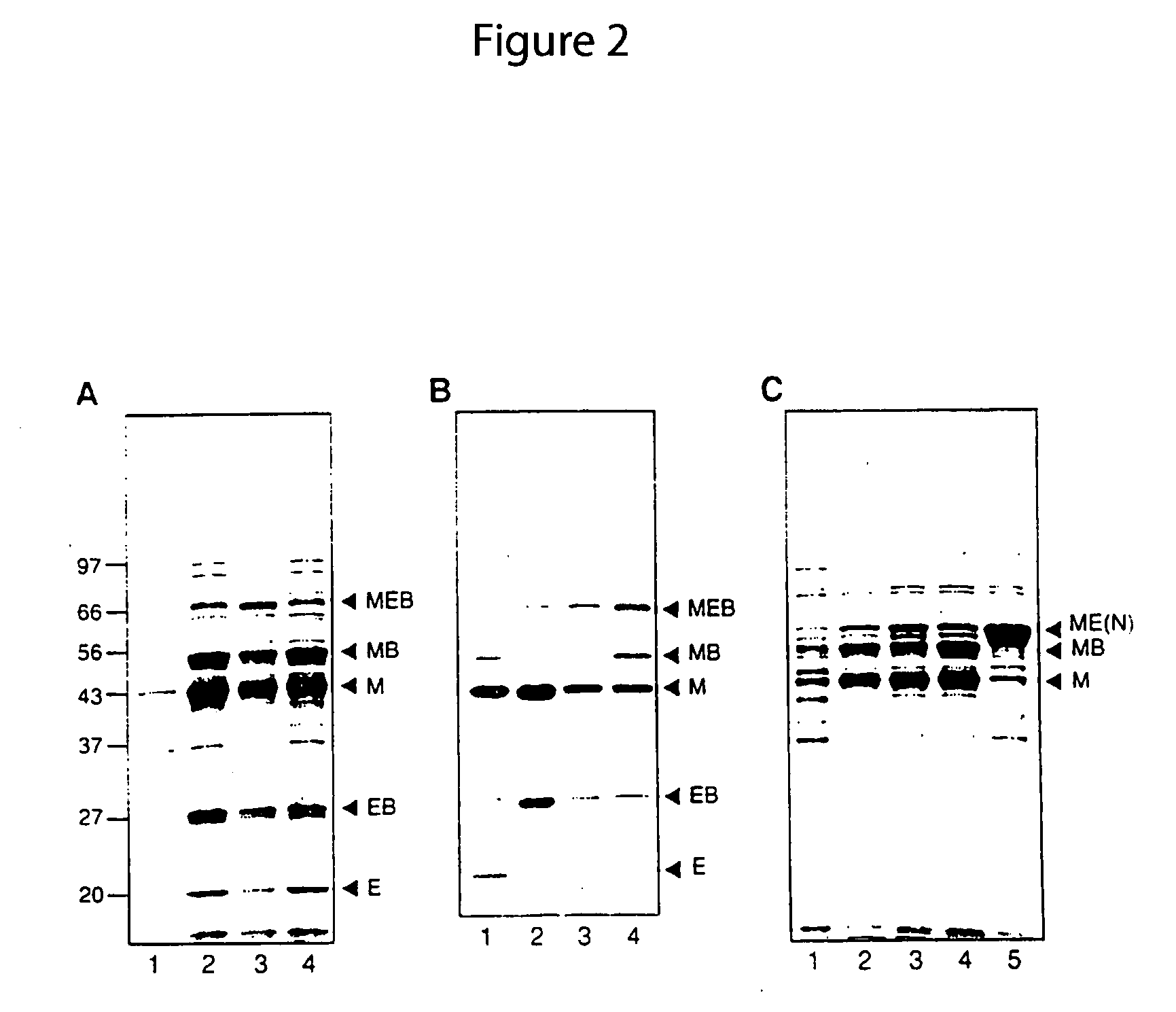
[0063]The in vivo splicing activity of the full length Ssp DnaE intein was investigated by analyzing protein expression from E. coli ER2566 cells (Chong, et al., Gene 192(2):271-281 (1997)) bearing plasmid pMEB8. The cells bearing the plasmid were grown in LB medium containing the appropriate antibiotic selection at 37° C. with shaking to an OD600 of 0.5. Protein expression was induced by the addition of 0.3 mM Isopropyl β-D-Thiogalactopyranoside (IPTG) at either 15° C. for 16 h or at 37° C. for 2 h. Crude cell extracts were visualized by electrophoresis on a 12% Tris-Glycine gel (Novex, San Diego, Calif.) followed by staining with Coomassie Brilliant Blue.
example iii
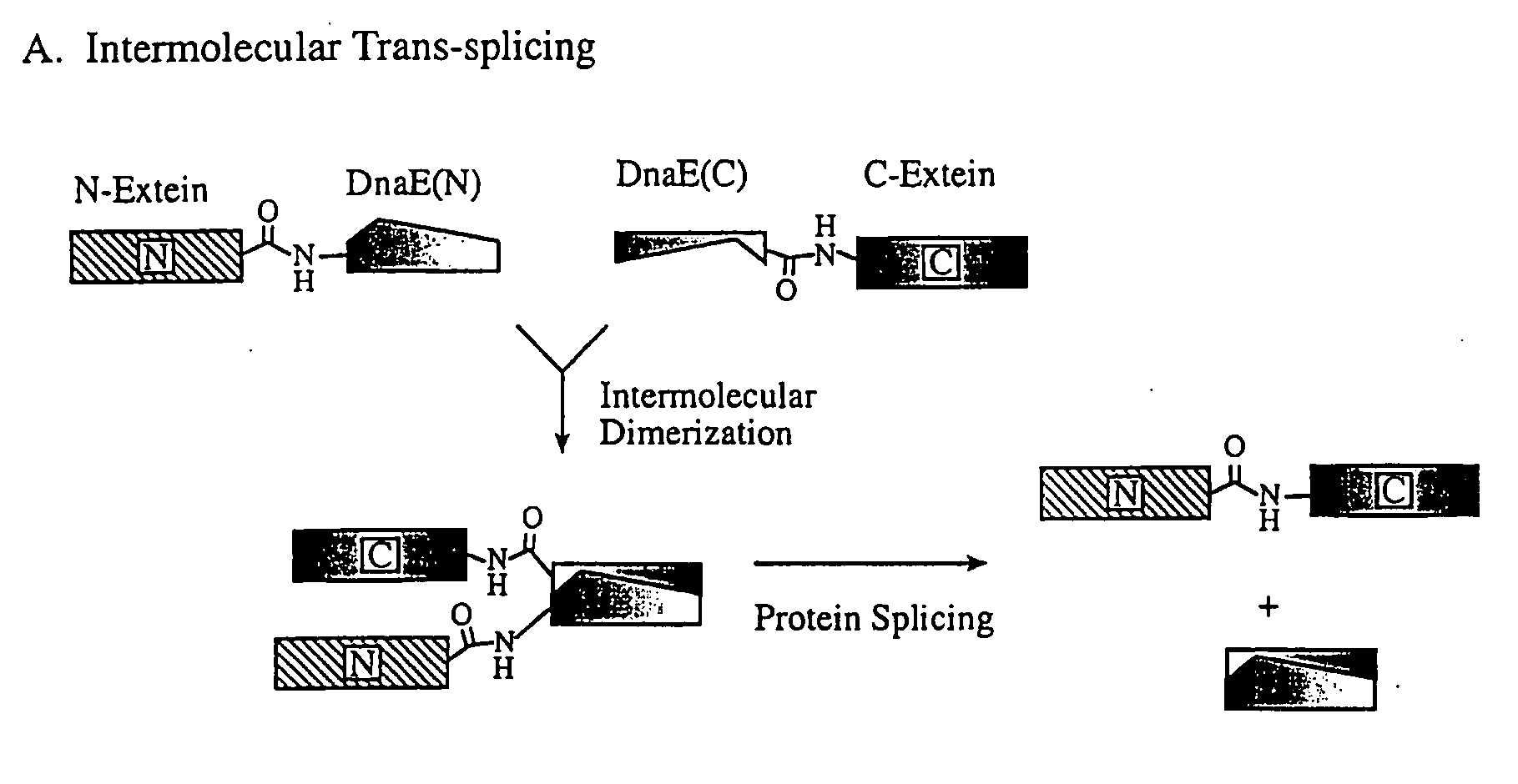
The Ligation of Two Protein In Vivo Using Trans-Splicing
[0064]The in vivo trans-splicing activity of the Ssp DnaE intein was demonstrated by analyzing the proteins from E. coli strain ER2566 (Chong, et al., Gene 192(2):271-281 (1997)) bearing the two compatible plasmids, pMEB4 and pKEB1. The E. coli bearing the plasmids were grown in LB medium containing the appropriate antibiotics (50 μg / mL kanamycin and 50 μg / mL ampicillin) at 37° C. with shaking until an OD600 of 0.5 was reached. Protein expression was induced by the addition of 0.3 mM Isopropyl β-D-Thiogalactopyranoside (IPTG) at either 15° C. for 16 h or at 37° C. for 2 h. Crude cell extracts were visualized by electrophoresis on a 12% Tris-Glycine gel (Novex, San Diego, Calif.) followed by staining with Coomassie Brilliant Blue.
Protein Purification for in vitro trans-splicing or trans-cleavage reactions:
[0065]ER2566 cells containing pMEB2 or pBEL11 were grown at 37° C. to an OD600 of 0.5. Following IPTG (0.5 mM) induced protei...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com