Multiloop Engineered Heart Muscle Tissue
a multi-ring engineered, heart muscle technology, applied in the direction of skeletal/connective tissue cells, embryonic cells, biomass after-treatment, etc., can solve the problems of insufficient endogenous regenerative mechanisms, inability to compensate for total organ failure, and engineering is still in its infancy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ETH Construction From Cardiac Myocytes of Neonatal Rats
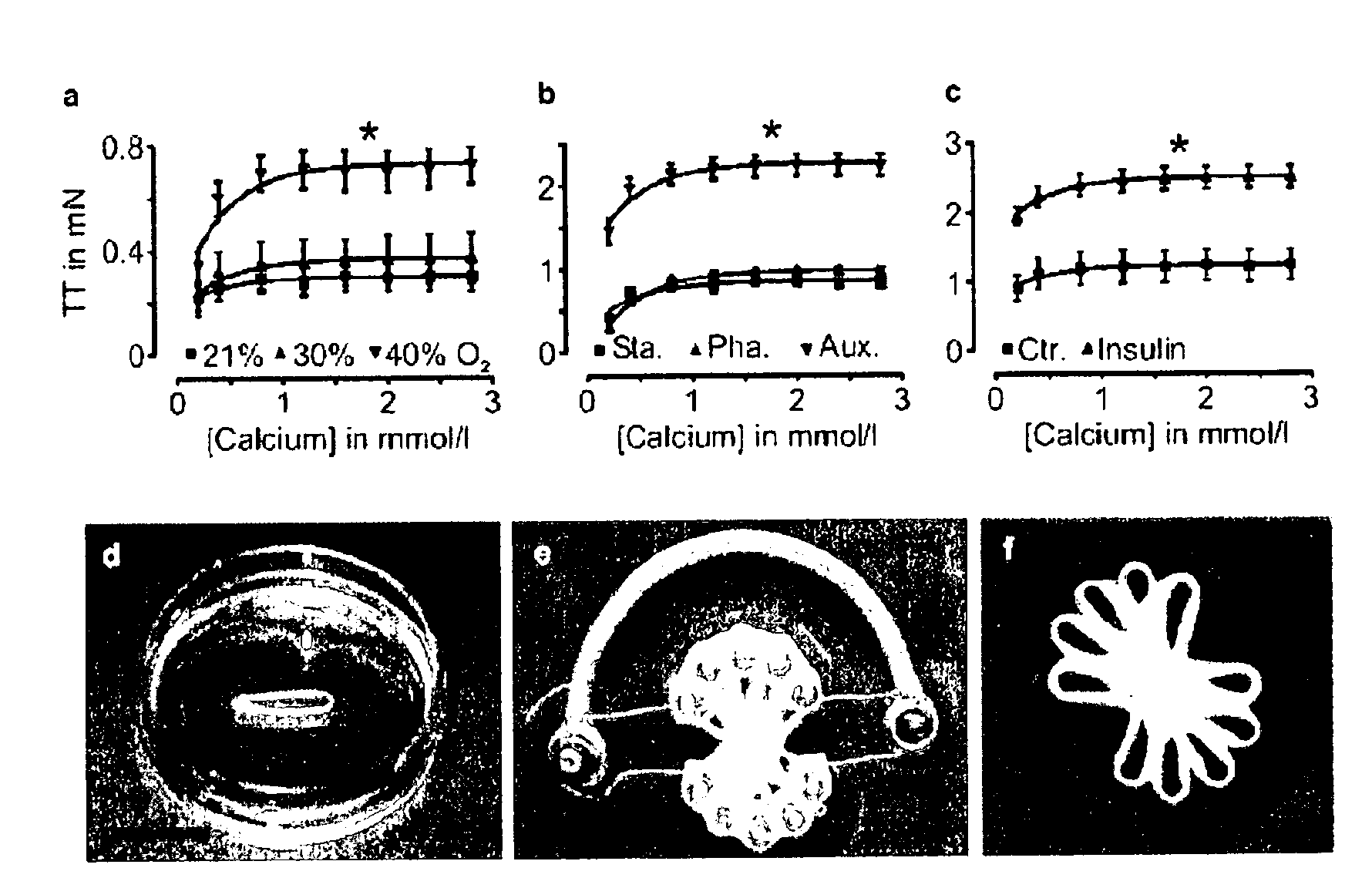
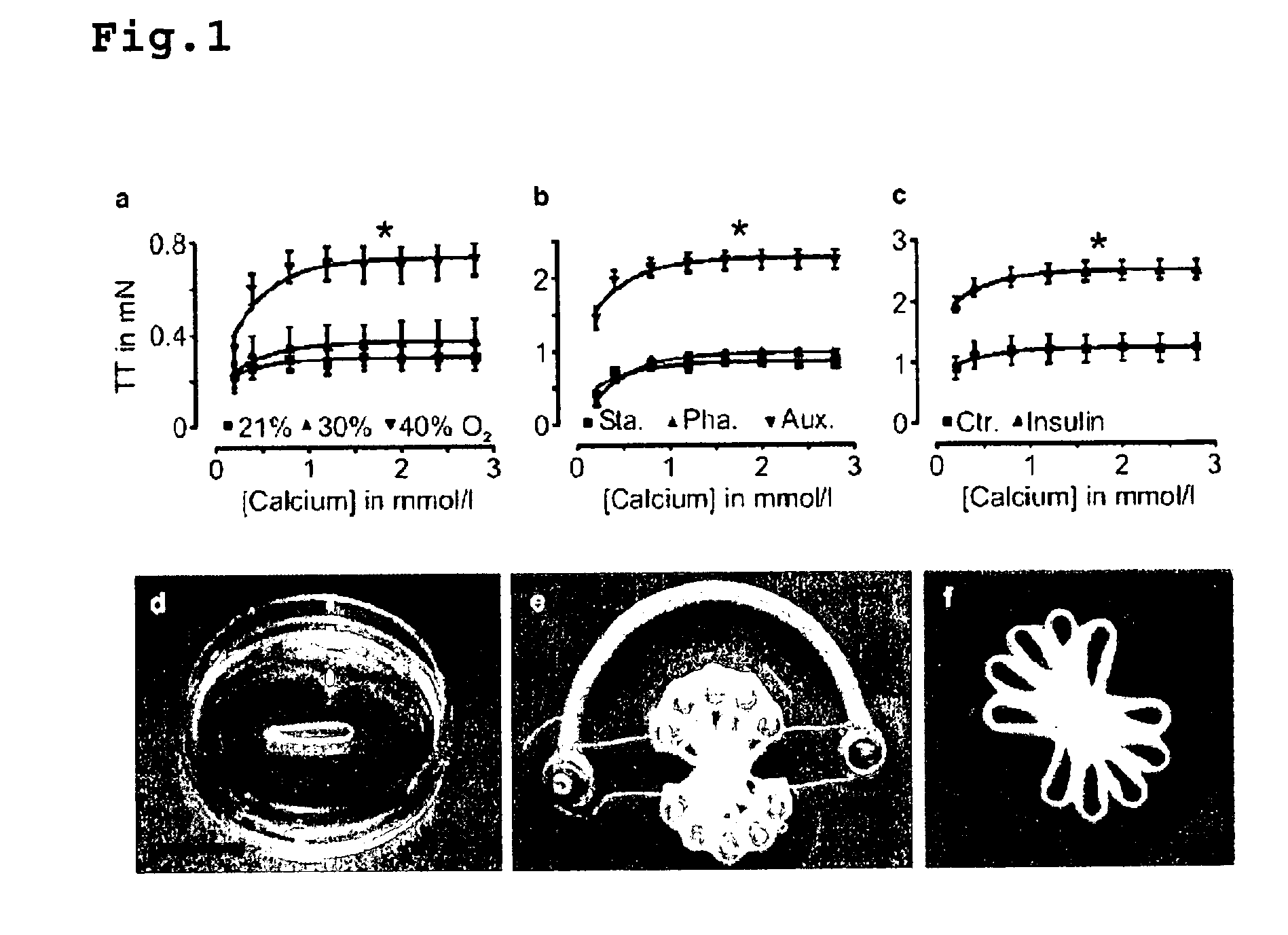
[0073]EHTs were constructed as previously described in Zimmermann, W. H. et al., Circulation 106, I 151-7 (2002) and published International Application WO 01 / 55297. Briefly, EHT rings (reconstitution volume: 0.9 ml) were prepared by mixing isolated heart cells from neonatal rats (2.5×106 cells / EHT) with collagen type I from rat tails (0.8 mg / EHT; pH adjusted to physiologic values ˜7.4 with 0.1 N NaOH), serum-containing culture medium (2×DMEM, 20% horse serum, 4% chick embryo extract, 200 U / mL penicillin, and 200 mg / mL streptomycin; similar volume as neutralized collagen), and Engelbreth-Holm-Swarm tumor exudate (“Matrigel” final concentration 10% v / v; tebu, France).
[0074]EHTs were transferred after 7 days of culturing in casting molds onto custom made stretch devices to facilitate static (110% of slack length), phasic (from 100 to 110% of slack length at 2 Hz), or auxotonic (culture on deflection coils at 110% of slack length a...
example 2
Infarct Model and Grafting
[0076]The potential of multiring EHT constructs to repair diseased hearts in male Wistar rats with myocardial infarcts was tested. Myocardial infarctions were generated in ventilated, isoflurane (2%) anesthetized male Wistar rats (318±3 g; n=121) by permanent ligation of the left anterior descending coronary artery (LAD ligation; 5-0, Prolene, Ethicon, Germany). 14 days after LAD ligation (first surgery) an independent and blinded investigator evaluated infarct localization and size by Echocardiography (ECHO).
[0077]Echocardiography was performed in volatile isoflurane (2%) anaesthesia as described previously with a HP Sonos 7500 System (Philips, Amsterdam, The Netherlands) equipped with a 15 MHz linear array transducer (Zimmermann, W. H. et al., Circulation 106, I 151-7 (2002)). To monitor changes in myocardial performance all animals were subjected to ECHO 14 days post LAD ligation and again 4 weeks after grafting or Sham-operation (longitudinal study desi...
example 3
Epicardial Mapping
[0080]Electrical coupling of multiring EHT constructs to the host myocardium was assessed 4 weeks after engraftment by high resolution epicardial mapping as described in Dhein, S. et al., Circulation 87, 617-30 (1993). Briefly, hearts were excised and Langendorff-perfused with Tyrode's solution at a constant pressure of 70 cm H2O and 37° C. Unipolar ECGs were recorded simultaneously from 256 AgCl electrodes (interelectrode distance: 1 mm; sampling rate: 4 kHz / electrode; HAL4 system; P. Rutten, Hamburg, Germany) arranged in 4 polyester blocks around the circumference of spontaneously beating hearts. Activation time was determined at each electrode and the spread of excitation was analyzed by constructing isochrones. For quantitative analysis the total activation time assessed as the delay between activation of the first and activation of the last electrode for each region under investigation was determined.
[0081]Sham animals (n=5) demonstrated the expected delay of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| outer diameter | aaaaa | aaaaa |
| twitch tension | aaaaa | aaaaa |
| twitch tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com