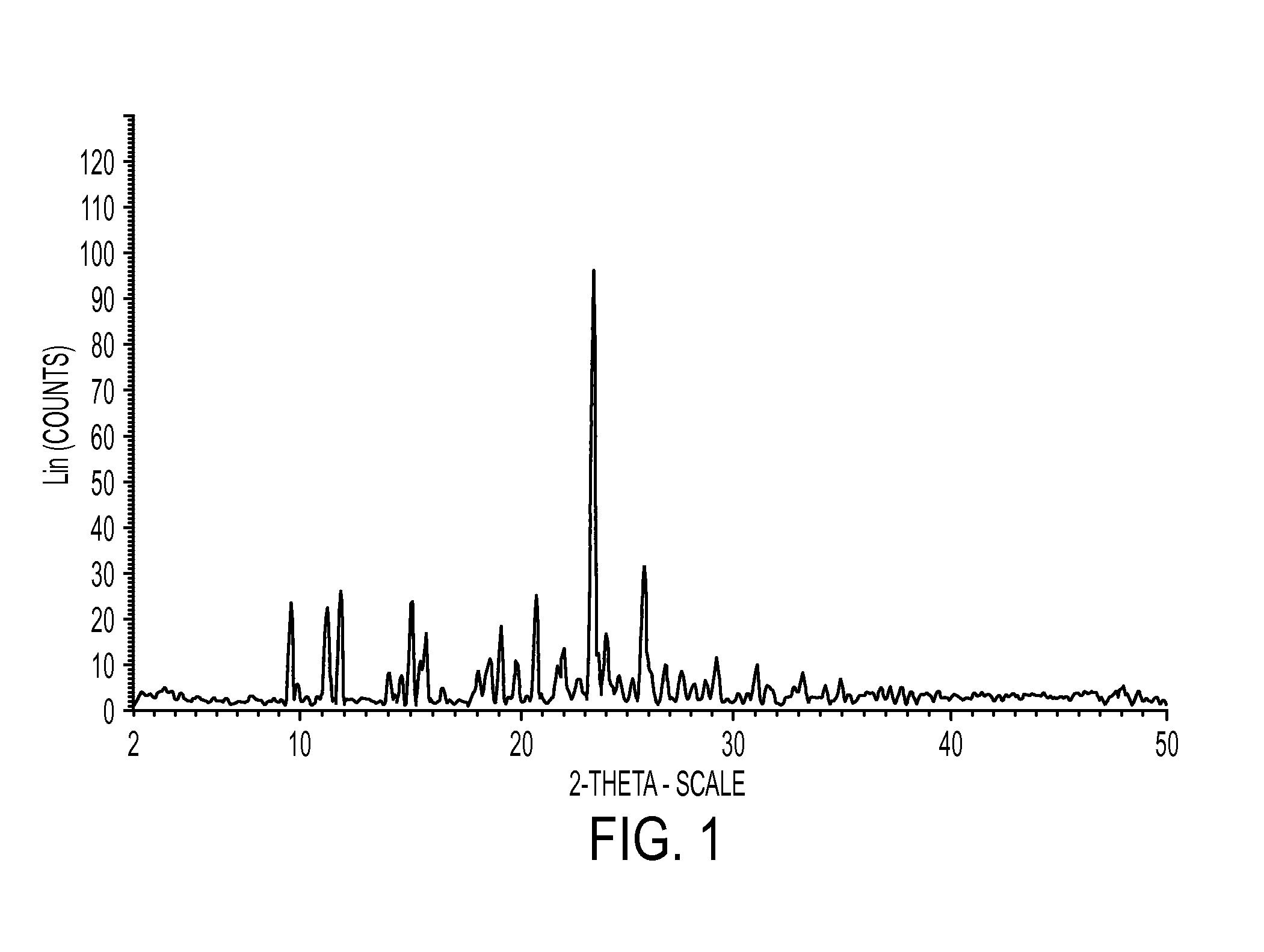
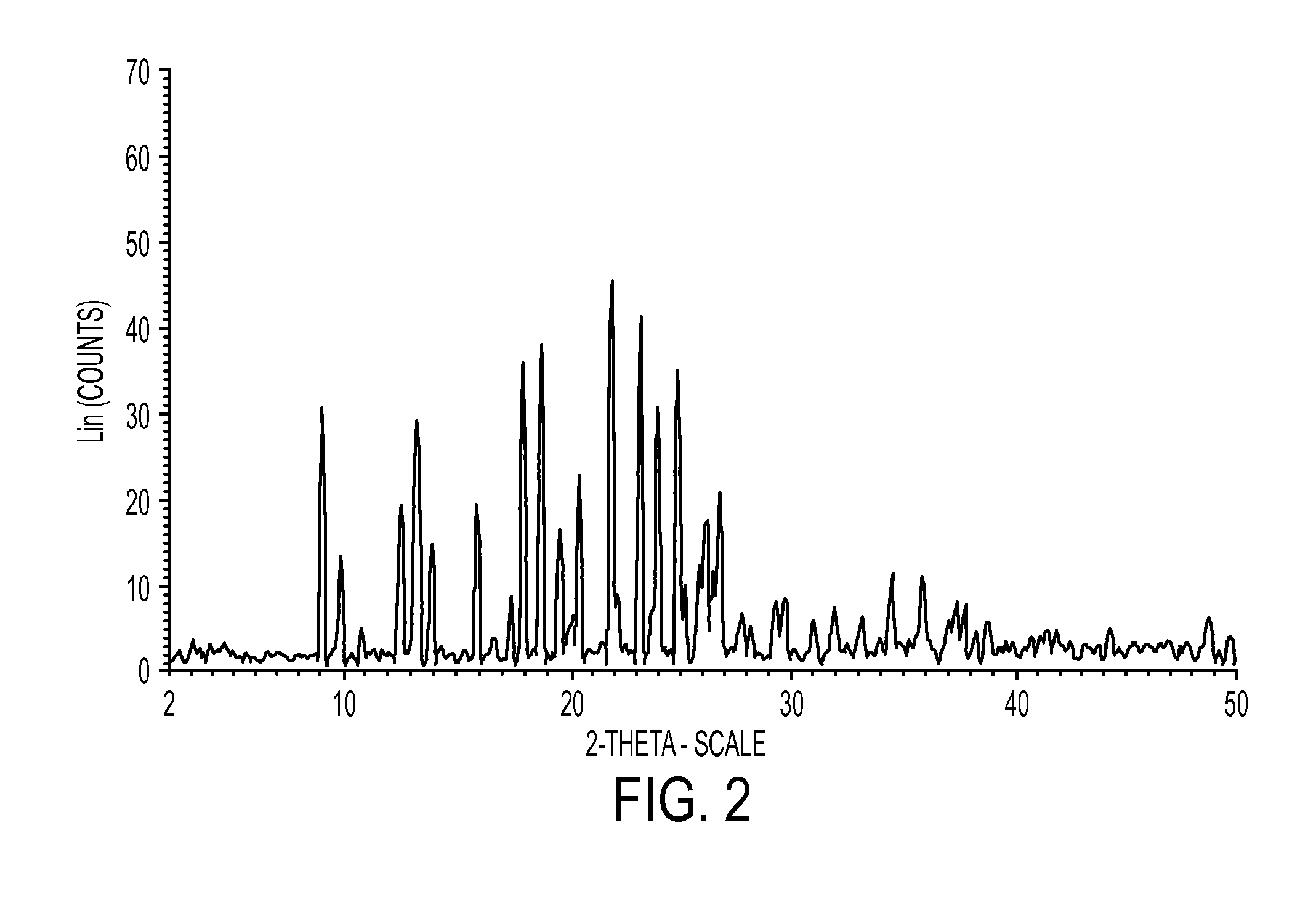
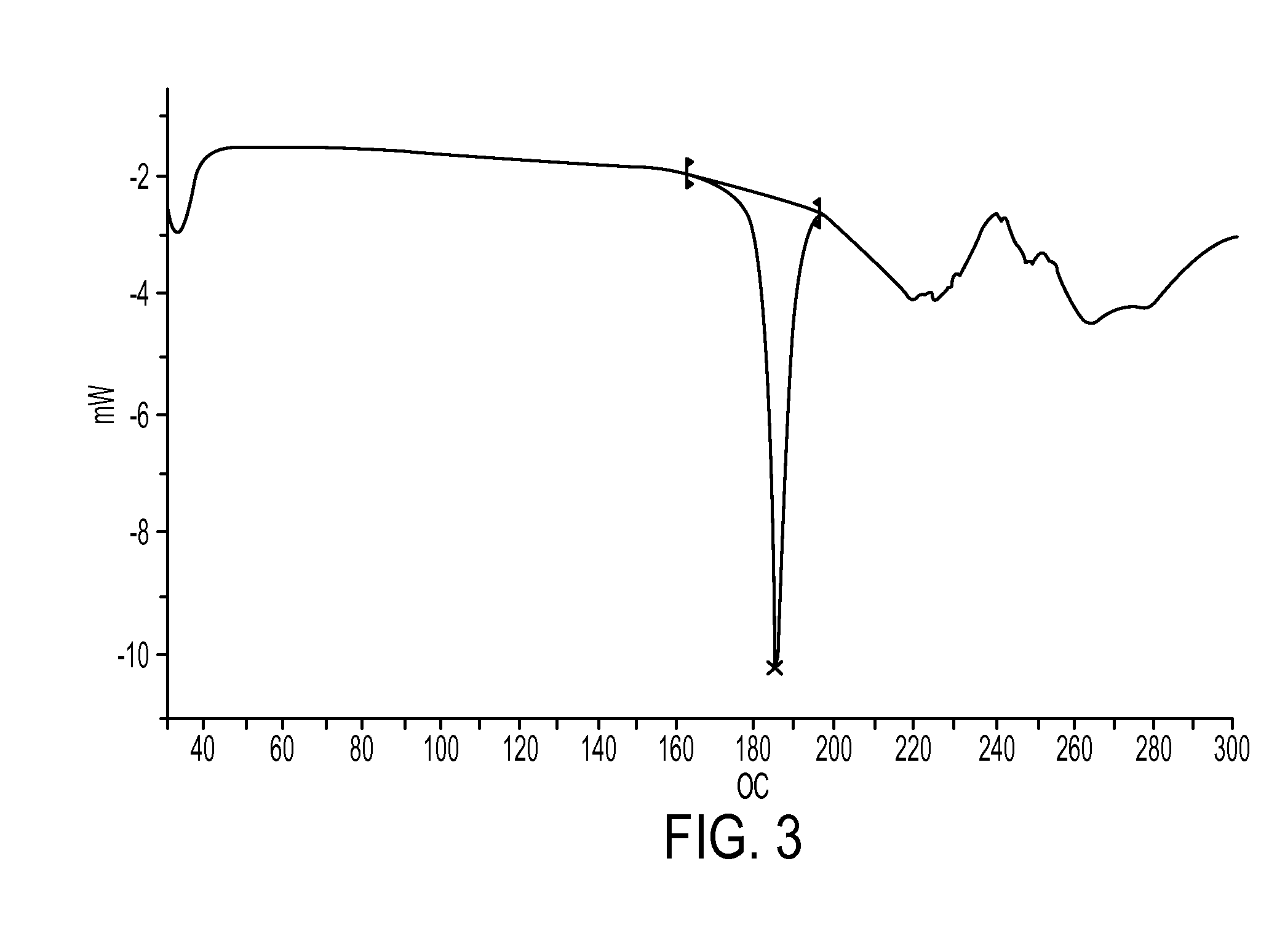
Industrial process for preparation of clopidogrel hydrogen sulphate
a technology of clopidogrel and hydrogen sulphate, which is applied in the field of industrial process for preparation of clopidogrel hydrogen sulphate, can solve the problems of unfriendly process plants, increased operation costs, and unsatisfactory effects of process plants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0102]One pot process for 4,5,6,7-tetrahydrothieno[3,2-c]pyridine hydrochloride 100 gm. of 2-thienylethylamine was charged in a 1 litre reaction vessel equipped with a dean stark assembly for azeotropic removal of water. Dichloroethane (600 ml.) was added and the mixture stirred for 5 minutes. 26.4 gm. paraformaldehyde was added and the reaction mass was heated to reflux. Water formed in the reaction was continuously removed. After 4 hours the reaction mass was cooled to 30° C. and 133 ml. of 6.6N hydrochloric acid solution in dimethyl formamide was added. The reaction mass was heated to 70° C. for 4 hours. The reaction cooled to 15° C. and filtered under suction and washed with dichloroethane. The solid obtained was dried in oven at 50° C. 124 gm. (90%) of 4,5,6,7-tetrahydrothieno[3,2-c]pyridine hydrochloride are obtained.
example 2
[0103]Clopidogrel base and clopidogrel hydrogen sulphate (dichloroethane as solvent). 50 gm. 4,5,6,7-tetrahydrothieno[3,2-c]pyridine hydrochloride was charged in 1 litre reaction vessel. 150 ml. dichloroethane was added and stirred for 5 minutes. 75 gm. of methyl-1-bromo-(2-chlorophenyl)acetate and 80 ml. triethyl amine was added. Stirred at 25° C. for 1 hour and then heated to reflux for 4 hours. The reaction mixture cooled to room temperature and quenched in water. The organic layer was washed with water, and distilled the dichloroethane to obtain clopidogrel base as an oil.
[0104]This clopidogrel base was dissolved in 300 ml. acetone and mixed with 17.5 ml. Conc. Sulphuric acid under cooling. The precipitated pure Clopidogrel hydrogen sulphate was filtered and washed with acetone. The precipitate was dried in an oven at 50° C. and 105 gm. (88%) Clopidogrel hydrogen sulphate was obtained.
example 3
Clopidogrel Base and Clopidogrel Hydrogen Sulphate (Water and Dichloroethane as Reaction Medium)
[0105]50 gm. of 4,5s637-tetrahydrothieno[3,2-c]pyridine hydrochloride was charged in 1 litre reaction vessel containing 500 ml. water and 75.4 gm. sodium carbonate and stirred for 1 hour. 75 gm. of methyl-1-bromo-(2-chlorophenyl)acetate in 250 ml. dichloroethane was added, stirred at 25° C. for 8 hours. The organic layer was separated and washed with water, and distilled the dichloroethane to obtain Clopidogrel base as an oil.
[0106]This was dissolved in acetone (300 ml.), cooled to 0-5° C. and mixed with 17.5 ml. conc. Sulphuric acid under cooling. The precipitated pure Clopidogrel hydrogen sulphate was filtered and washed with acetone. The precipitate was dried in an oven at 50° C. The 105 gm. (88%) Clopidogrel hydrogen sulphate was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com