Host Cells Used For Production of Recombinant Protein
a technology of host cells and recombinant proteins, which is applied in the direction of genetically modified cells, peptide/protein ingredients, peptide sources, etc., can solve the problems of enormous time required for obtaining the gene product (protein), difficult to produce functional proteins from microorganisms, and low amount of production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation of Cat Renal Tubular Epithelial Cells
[0045]Cat distal tubule-derived epithelial cells were isolated as follows according to the method described in Soshiki Baiyo no Gijyutsu (2nd edit) Nihon Soshiki Baiyo Gakkai Hen, Asakura Shoten, 7-4 Jinsaibo Baiyo Ho (pp. 141-145) (issue Culture Techniques (2nd edit) edited by the Japanese Tissue Culture Association, Asakura Shoten, 7-4 Renal Cell Culture Method (pp. 141-145)). A cat was subjected to anesthesia, and the kidney was aseptically excised. The excised kidney was fragmented with a knife in a Joklik buffer (111 mM NaCl, 24 mM NaHCO3, 10 mM Na2HPO4, 5.4 mM KCl, 1 mM Mg2SO4, and 11 mM glucose). 30 ml of a 0.02% collagenase digestive fluid was added to 1 g of the fragmented tissue section in a 100-ml Erlenmeyer flask, and the obtained mixture was then stirred with a stirrer for 30 minutes. Thereafter, a supernatant was discarded, and a fresh digestive fluid was added, followed by a further reaction for 30 minutes. The fragmented...
example 2
Establishment of FKD Cells
[0046]Using Lipofectamine-Plus (Invitrogen), pSV3-neo (ATCC37150) was transfected to the distal tubule-derived cells isolated in Example 1. Forty-eight hours after the transfection, the medium was exchanged with a G418-containing medium, and a stable mutation introduced cell line was then selected. The obtained FKD cells are shown in FIG. 1. The proliferated cells were subjected to a passage, and the resultant cells were then preserved in liquid nitrogen.
example 3
Subculture (Passage) of FKD Cells
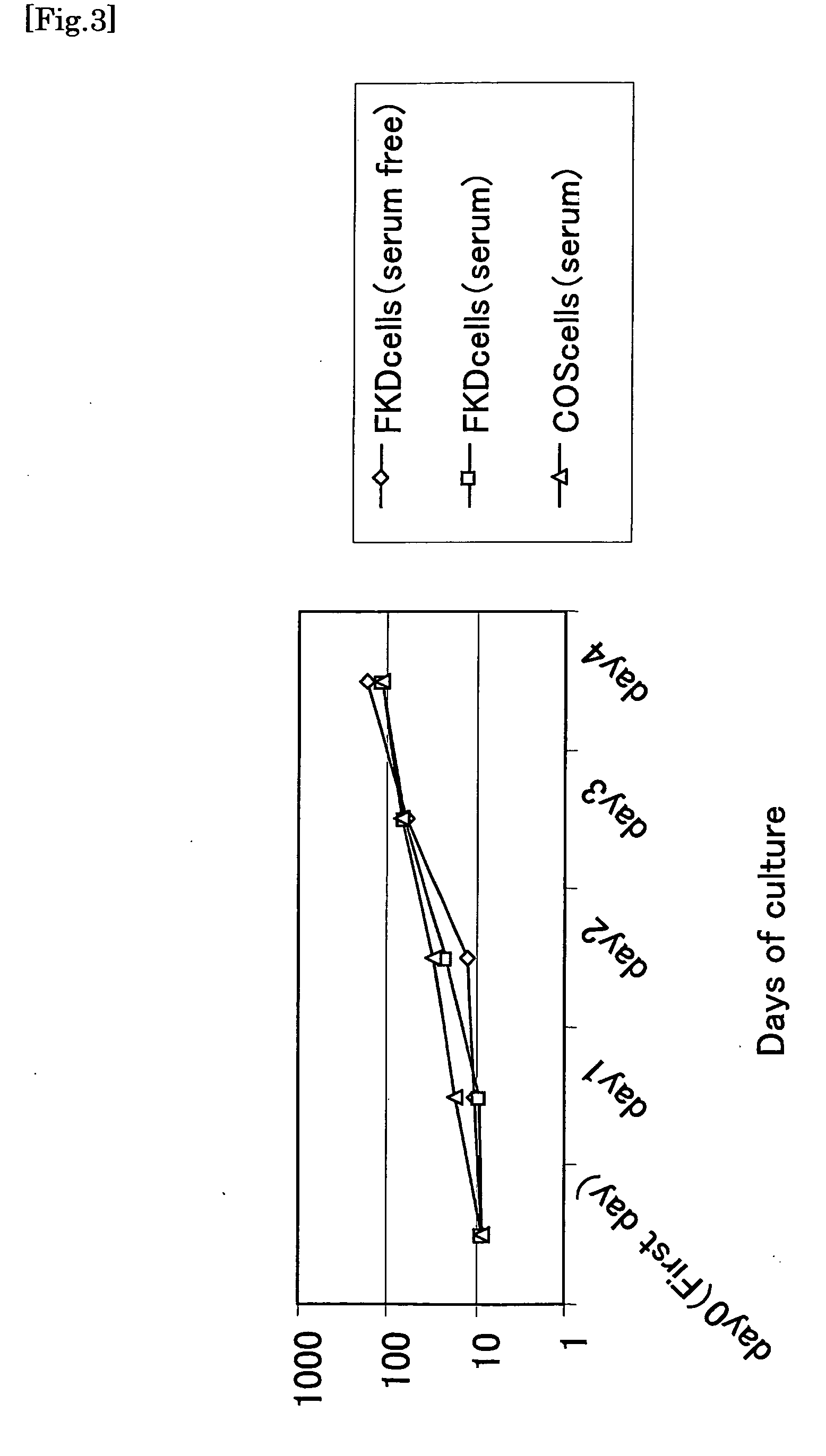
[0047]FKD cells were subcultured in a DMEM-F12 (+supplement) medium on a collagen-coated dish at 37° C. in the presence of 5% CO2. When the cells became 80% to 90% confluent, the medium was removed. The cells were washed with PBS / EDTA, and 1 ml of PBS / EDTA was then added thereto, followed by incubation at 37° C. in the presence of 5% CO2 for 5 minutes. Subsequently, the cells were washed with 0.1% trypsin-PBS / EDTA, followed by incubation under the same above conditions. Thereafter, the resultant cells were suspended in a DMEM-F12 (+supplement) medium, and the suspension was diluted and then cultured in a fresh collagen-coated dish. As a supplement (GIBCO ITS-X supplement), a product comprising insulin, transferrin and serine was used.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com