Gastroresistant Pharmaceutical Formulations Containing Rifaximin
a technology of rifaximin and rifaximin, which is applied in the direction of drug compositions, antibacterial agents, dispersed delivery, etc., can solve the problems of negative impact on use, side effects and unwanted risks of pharmacological interactions, and the absence of a placebo group in clinical trials, so as to facilitate ingestion, wide modulation of dosages, and modulation of dose strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Rifaximin Preparation in Gastroresistant Microgranules
[0060]In a fluid bed apparatus, Glatt GPC 30, with a Wurster system of 18 inches with a 1.8 mm spray jet, 25000 g of rifaximin powder and 125 g of Aerosil as fluidiser are loaded. Contemporaneously in a mixer under agitation a suspension is prepared using 48107 g of demineralised water, 9281 g of methacrylic acid ethylacrylate copolymer marketed under the trademark KOLLICOAT® MAE 100 P, 1392 g propylglycol, 2475 g of talc, 557 g of titanium dioxide FU and 62 g of iron oxide E 172. The solid components of the suspension are homogeneously mixed in demineralised water with an high speed homogeniser (Ultra Turrax). The prepared suspension feeds the spray system of the fluid bed apparatus and nebulized, at a pressure between 1.0 and 1.5 bar, trough the 1.8 mm nozzle on the mixture of rifaximin powder and Aerosil 200 maintained in suspension in the fluid bed by a warm air flow.
[0061]The applied conditions are described in table 1:
TABLE...
example 2
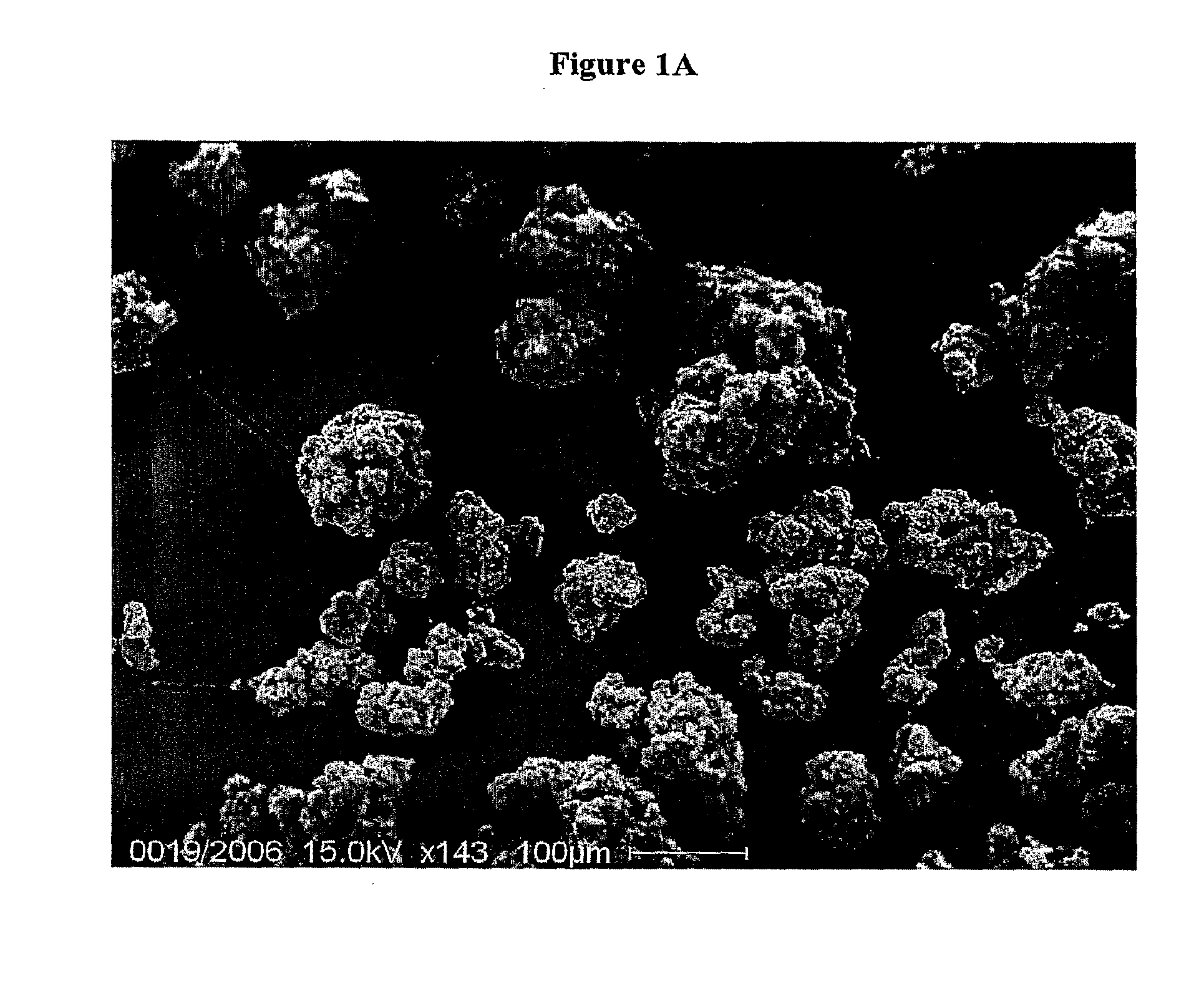
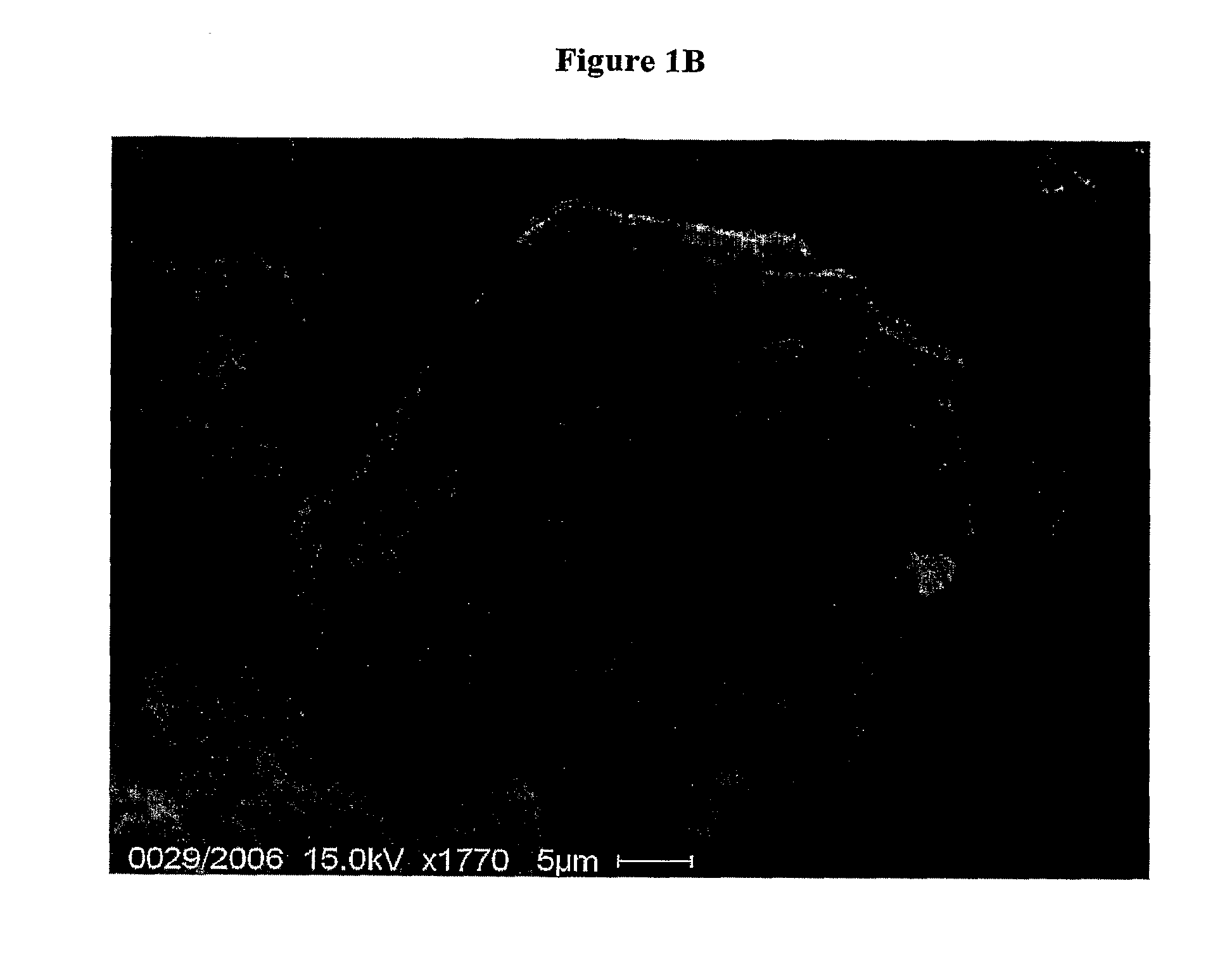
SEM Microscopy of Gastroresistant Microgranules of Rifaximin
[0068]A SEM Philips 515 instrument is used for the observations.
[0069]Rifaximin gastroresistant microgranules are sputtered with gold by current stream of 30 mA, getting an Au-layer of about 100 nm. An accelerating voltage of 15 kV is applied.
[0070]The images are digitally recorded with a CCD camera.
[0071]An image of microgranules of rifaximin is shown in FIG. 1A, while 20 in FIG. 1B a detail of a single microgranule is shown.
example 3
[0072]Gastroresistant Microgranules of Rifaximin Prepared in Thermo Welded Bags
[0073]9.12 Kg of gastroresistant rifaximin microgranules prepared according to the example 1, 19.58 Kg of sorbitol, 0.49 Kg of aspartame, 0.21 Kg of anhydrous citric acid, 2.10 Kg of pectin, 2.10 Kg of mannitol, 0.21 Kg of neohesperidine DC, 1.12 Kg of cherry flavour and 0.07 Kg of silica gel are sieved on a sieve with mesh of 0.5 mm and then mixed for 20 minutes in a V mixer. The resulting mixture is divided in thermo welded bags containing 5 grams of product corresponding to 800 mg of rifaximin. In the following Table 2 the composition of the medicinal speciality, thermo welded bag, is reported:
TABLE 2AmountComponents(mg)%Gastroresistant rifaximin microgranules130326.06(corresponding to 800 mg of rifaximin)Aspartame701.40Anhydrous citric acid300.60Pectin3006.00Mannitol3006.00Neohesperidin DC300.60Sorbitol279755.94Cherry-flavour1603.20Silica gel100.20
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com