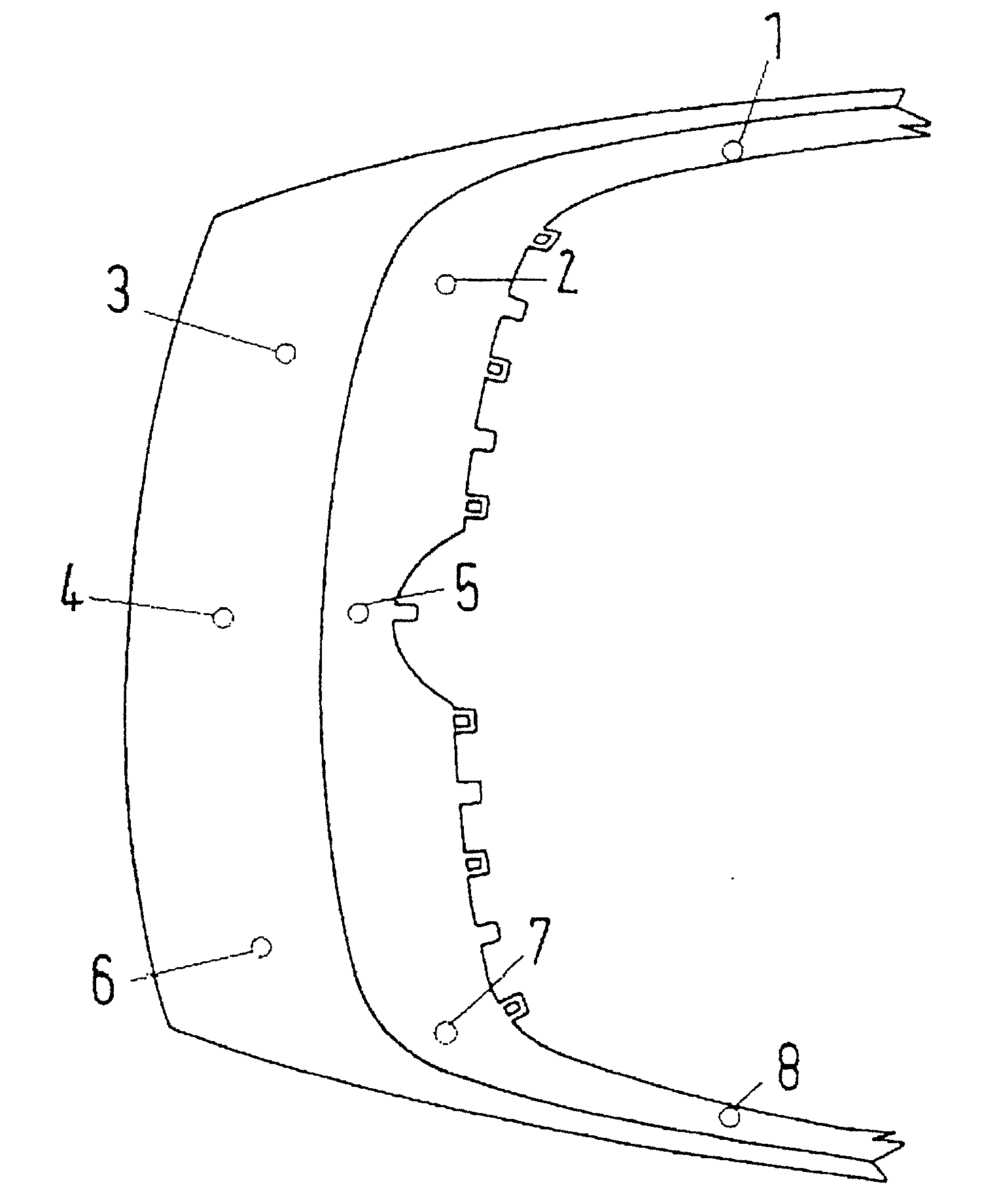
Method for Electrochemically Depositing a Metal on a Substrate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Preparation of Silica Particles Modified by Bonding an Aminosilane to the Surface Thereof
[0070]Syloid 244FP (Grace, peak volume 15.2% at 3.3 μm) was dried at 110° C. in an oven for two hours. The silica powder lost 4.2% weight. 5.0 ml (3-aminopropyl)triethoxysilane were dissolved in 100 ml chloroform (HPLC grade, water <0.001%) and poured over 15 g of the silica powder in a PP bottle which then was tightly closed. The opaque gel after short reaction time turned to a slurry. After one hour, the suspension was poured over a filter paper in a Büchner funnel, the chloroform was sucked off by vacuum and the remaining material was carefully washed with chloroform. The resulting material was again dried at 110° C. until its weight did not change anymore. The weight gain after the reaction was 9.5%.
example 2.1
[0071]The trial of example 2 was repeated with SD-530.
Initial weightOven temperatureDrying durationWeight loss[g][° C.][min][%]20.3000110604.8823.6294110905.40
Initial weight Reaction duration Weight gain [g][min][%]14.69482408.1716.3673 306.90
[0072]At different concentrations of the aminosilane, the weight gain obtained was only slightly different:
Amount of silane [ml]Weight gain [%]1.004.062.005.513.006.90
example 3
Preparation of Silica Particles Modified by Bonding an Aminosilane to the Surface Thereof and Use Thereof in a Nickel Electroplating Bath
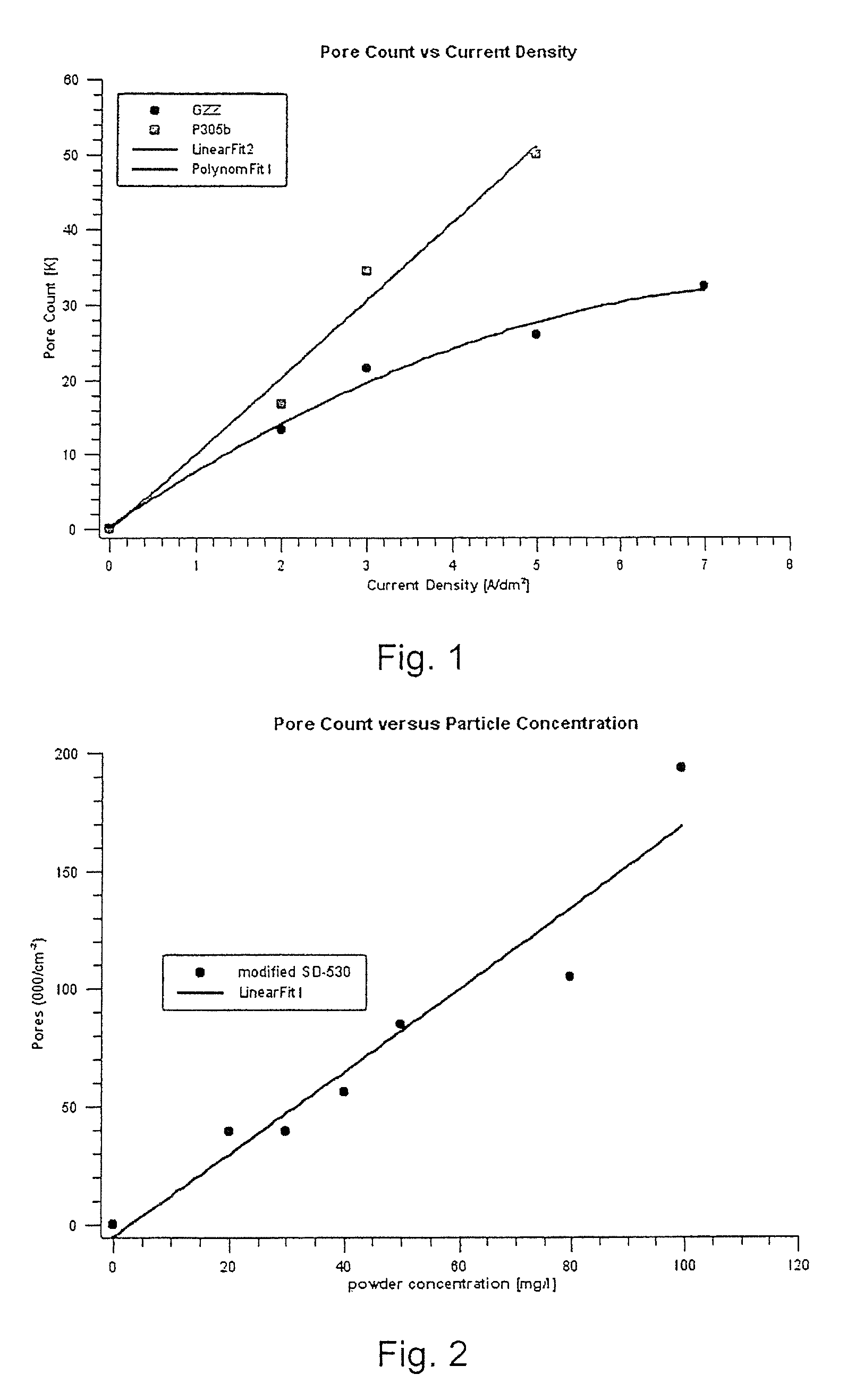
[0073]Two kinds of particles were compared: a modified SD-530 as described in example 1—but with 5 ml aminosilane for 15 g powder, and a commercially used alumina-modified silica. From a stock of 5 l Watts nickel electrolyte, one liter was adjusted with saccharine, surfactant and brightener as is already described in example 1. In a 250 ml Hull cell, the adjusted electrolyte was used to plate micro-porous nickel within three minutes at 2 A cell current. The amount of pores versus current was then calculated. FIG. 1 shows the relationship obtained of the pore count vs. current density for the electrolyte solution according to the invention (A) and for a prior art electrolyte solution containing alumina and silica particles which are not modified with an organic moiety according to the invention. While pore count made by the alumina-containing materi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com