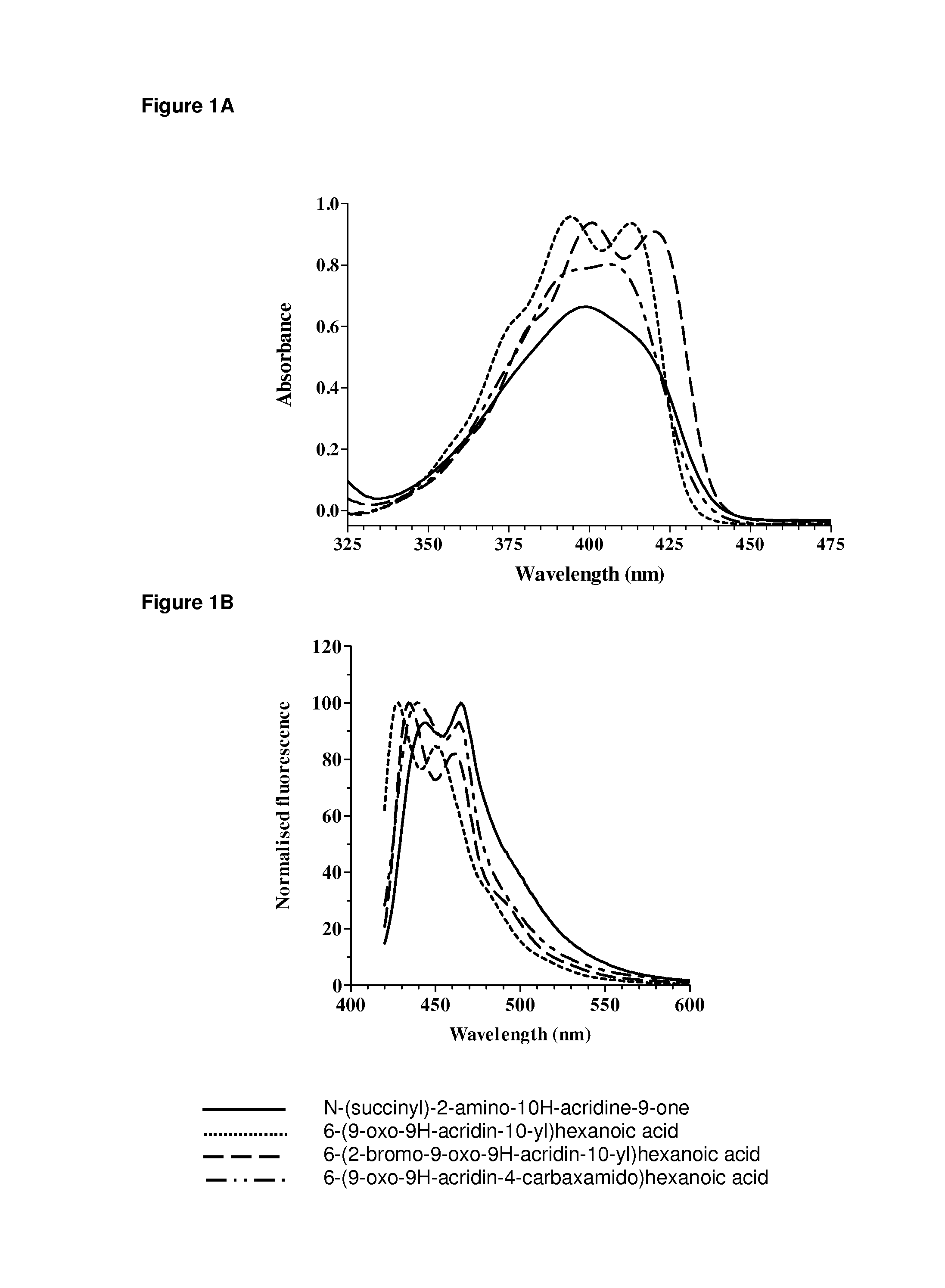
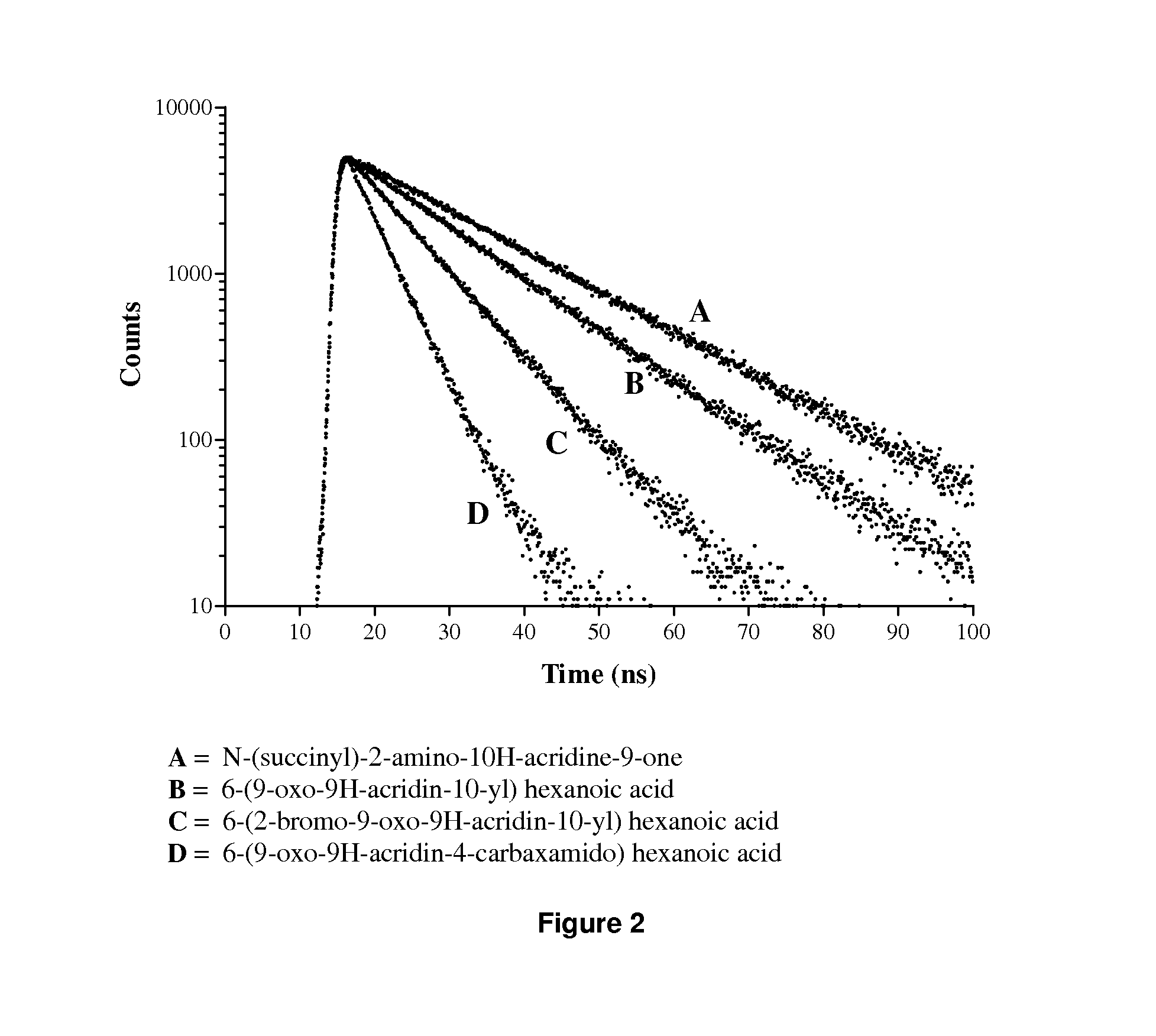
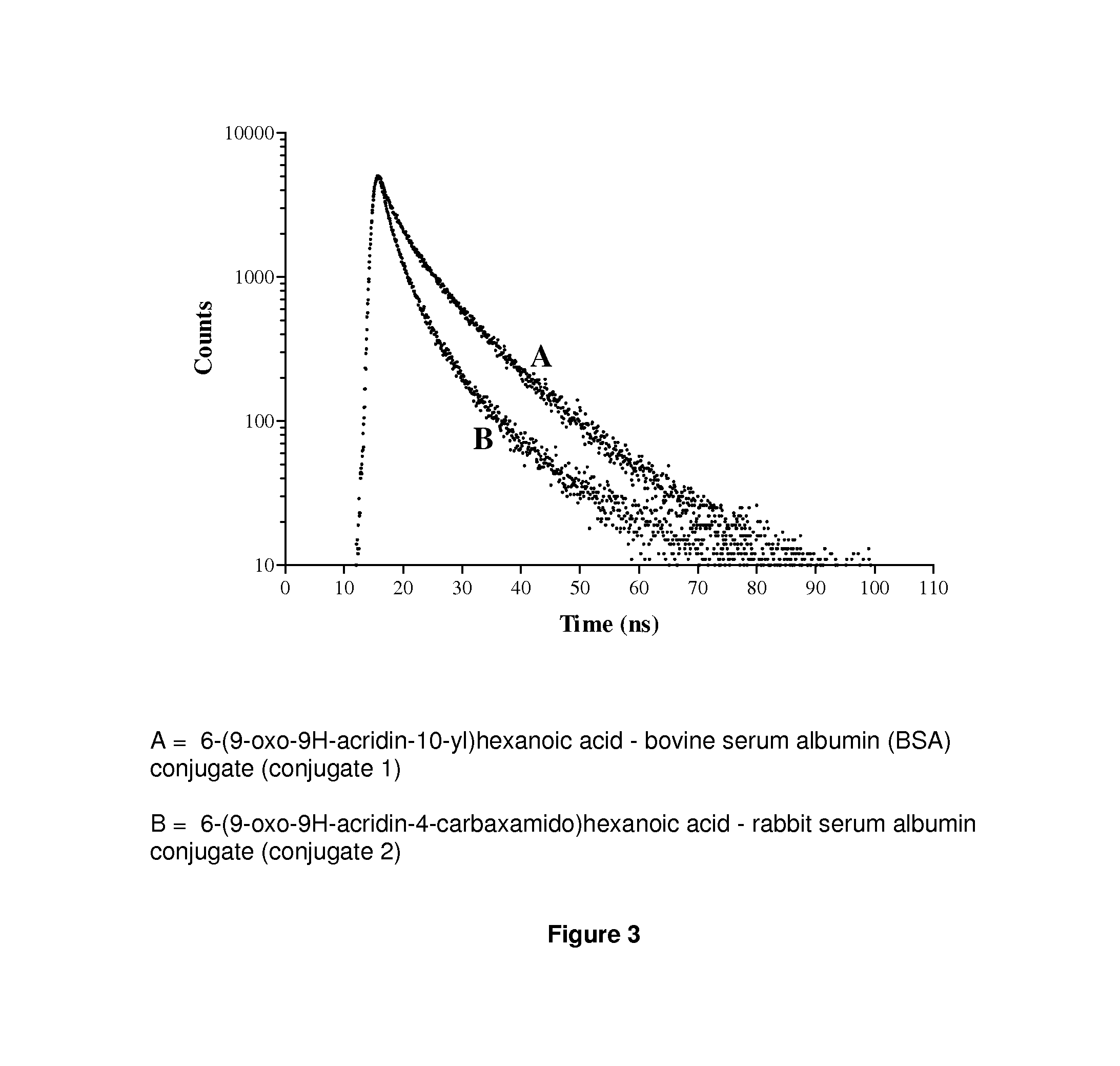
Acridone derivatives as labels for fluorescence detection of target materials
a technology of target materials and derivatives, applied in the field of acridone derivatives, can solve problems such as background fluorescence interference with measuremen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0110]The present examples are provided for illustrative purposes only, and are not to be construed as limiting the present invention as defined by the appended claims. All references given below and elsewhere in the present specification are hereby included herein by reference.
1. O—(N-Succinimidyl)-6-(9-oxo-9H-acridin-10-yl)hexanoate
[0111]
1.1 O-Ethyl-6-(9-oxo-9H-acridin-10-yl)hexanoate
[0112]9-(10H)-Acridone (4.88 g, 25 mmol) was mixed with anhydrous methyl sulfoxide (25 ml) under nitrogen atmosphere and set stirring. After 5 minutes, the resultant yellow slurry was treated with potassium tert-butoxide (3.37 g, 30 mmol) and stirring continued until all the solids had dissolved. Ethyl 6-bromohexanoate (6.7 g, 30 mmol) was then added and the resulting solution stirred under nitrogen for 3 days. At the end of this time the reaction mixture was poured into water (1000 ml) and extracted with ethyl acetate. The organic solution was washed with 0.5M aqueous HCl, then with water, before bei...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com