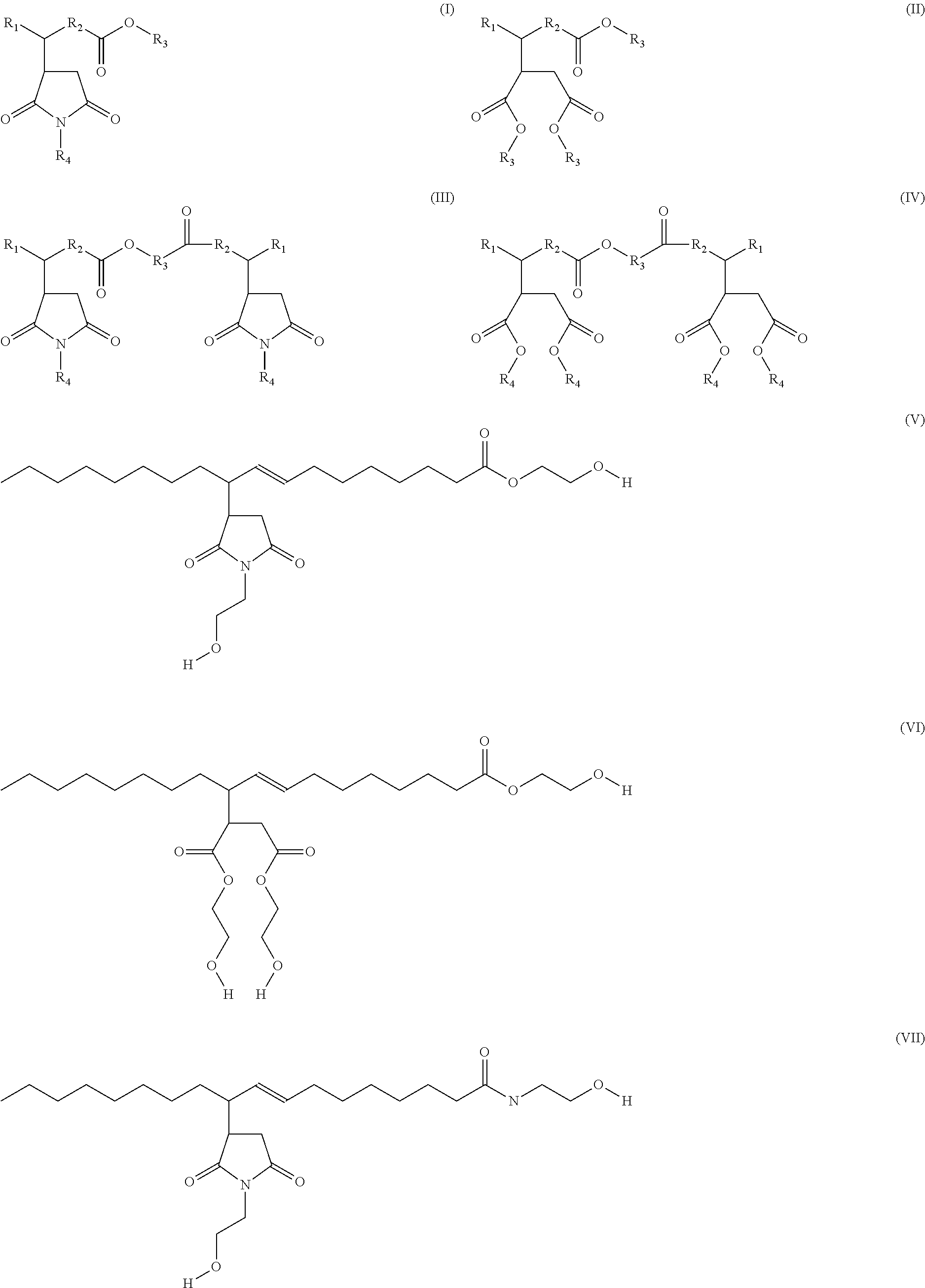Functionalized Maleated Fatty Acids as Non Acidic Fluid Additives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1-5
Preparation of Functionalized Maleated Fatty Acid Products
example 1
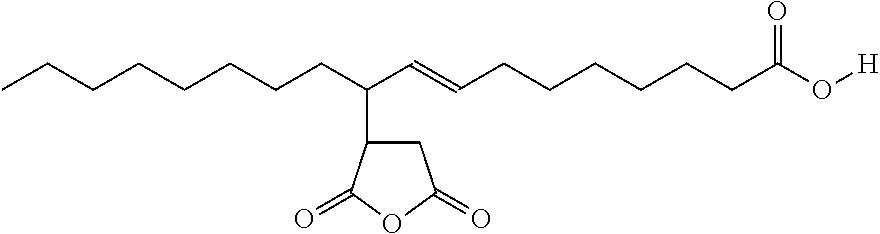
[0029]The preparation of maleated fatty acid: In a typical reaction, oleic acid (100 g) and maleic anhydride (25 g) were charged into a 250 ml 3-neck flask under nitrogen. The mixture was heated sequentially up to 240° C. for 30 hrs until the reaction was completed. The reaction was monitored by FT-infrared spectroscopy (FT-IR) as is known to those skilled in the art. The final product was diluted by aromatic solvent to a concentration of 90% active and marked as Example 1.
[0030]Above is a representative structure of Example 1 material.
example 2
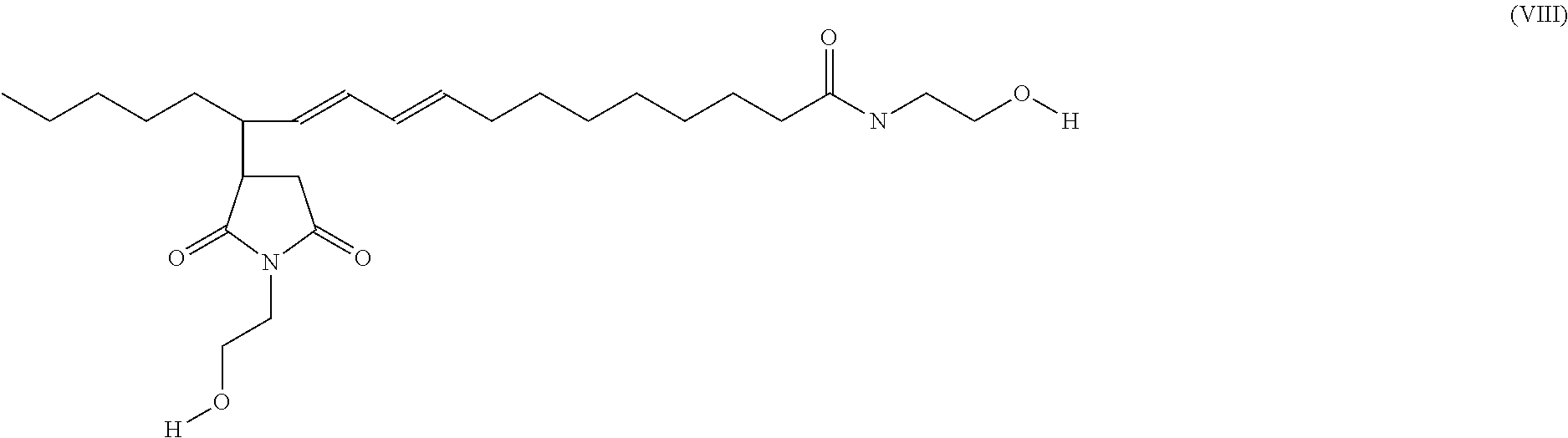
[0031]Oleic acid (100.1 g) and maleic anhydride (25.3 g) were mixed in a 3-neck flask. The mixture was heated sequentially up to 240° C. until the reaction is completed as monitored by FT-IR. The reaction mixture was first cooled to room temperature, and then ethanolamine (38.0 g) was added drop-wise while stirring. After the addition was completed, the reaction temperature was first set at 80° C., and then raised to 180° C. sequentially in 2 hours. The reaction process was monitored by FT-IR and acid number analysis. A second batch of ethanolamine (6.0 g) was added after 8 hrs reaction time. The reaction was stopped when the acid number is below 3. The final product was diluted with aromatic solvent to a concentration of 80% active and marked as Example 2.
[0032]Above is a representative structure of Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com