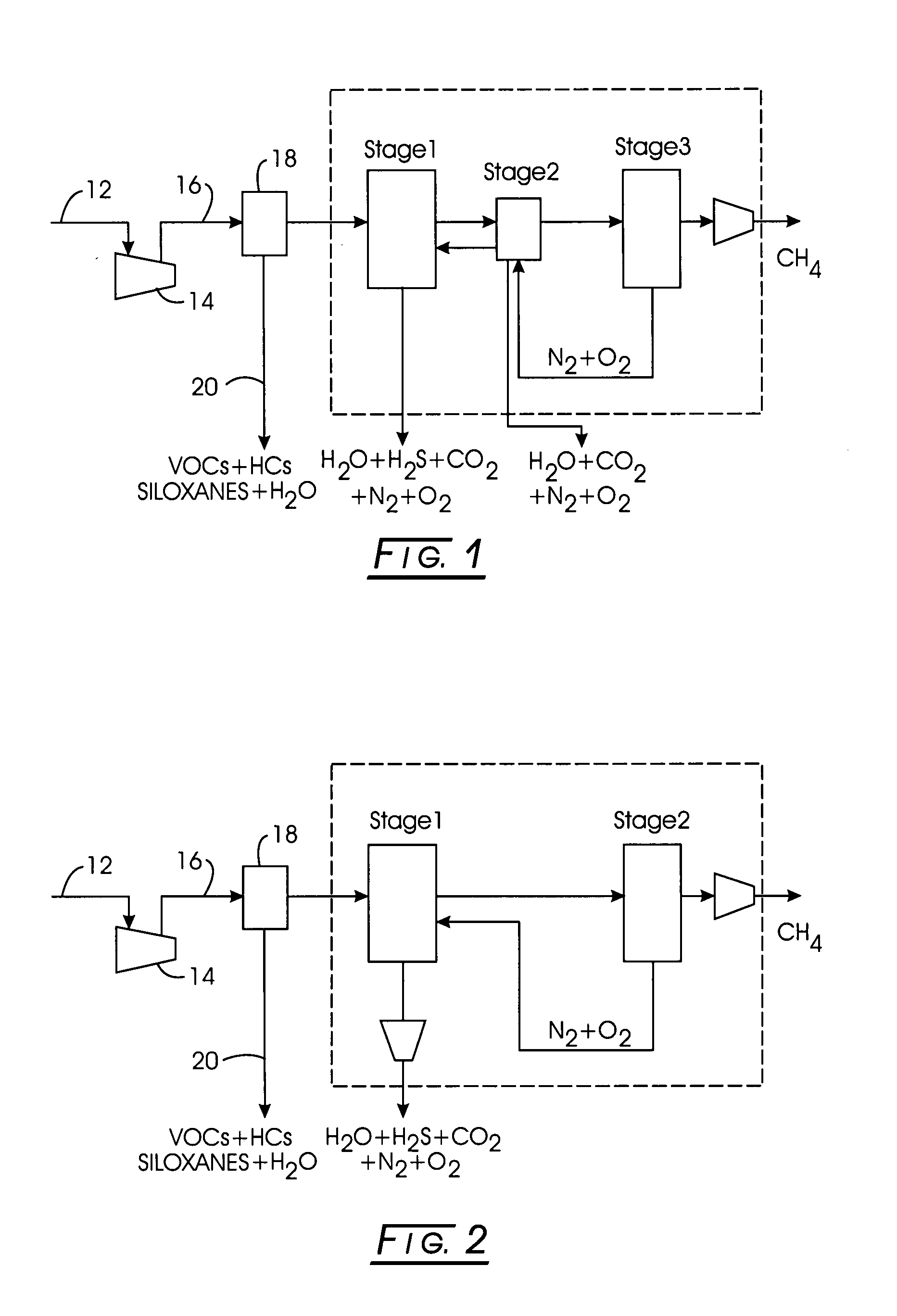
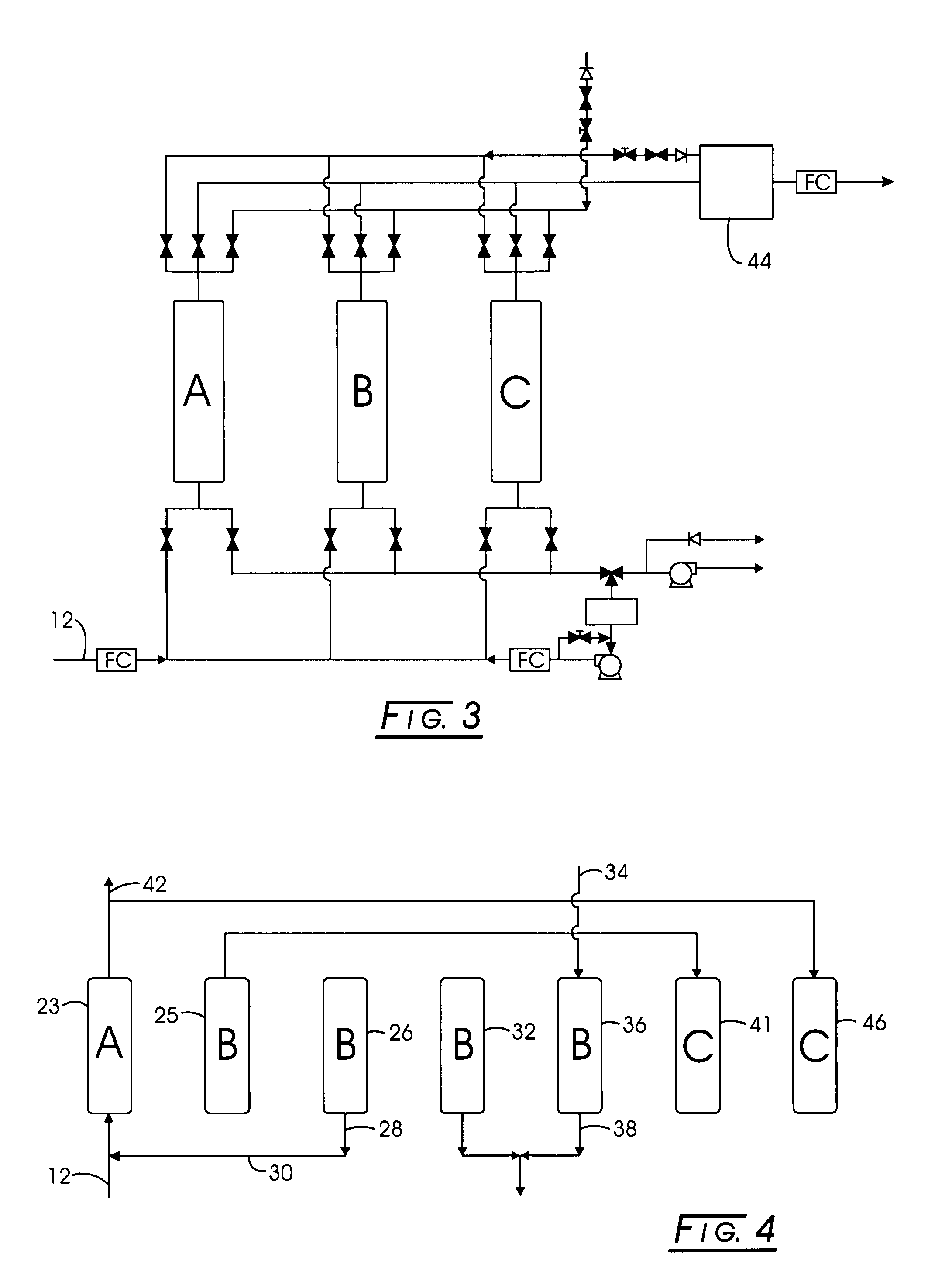
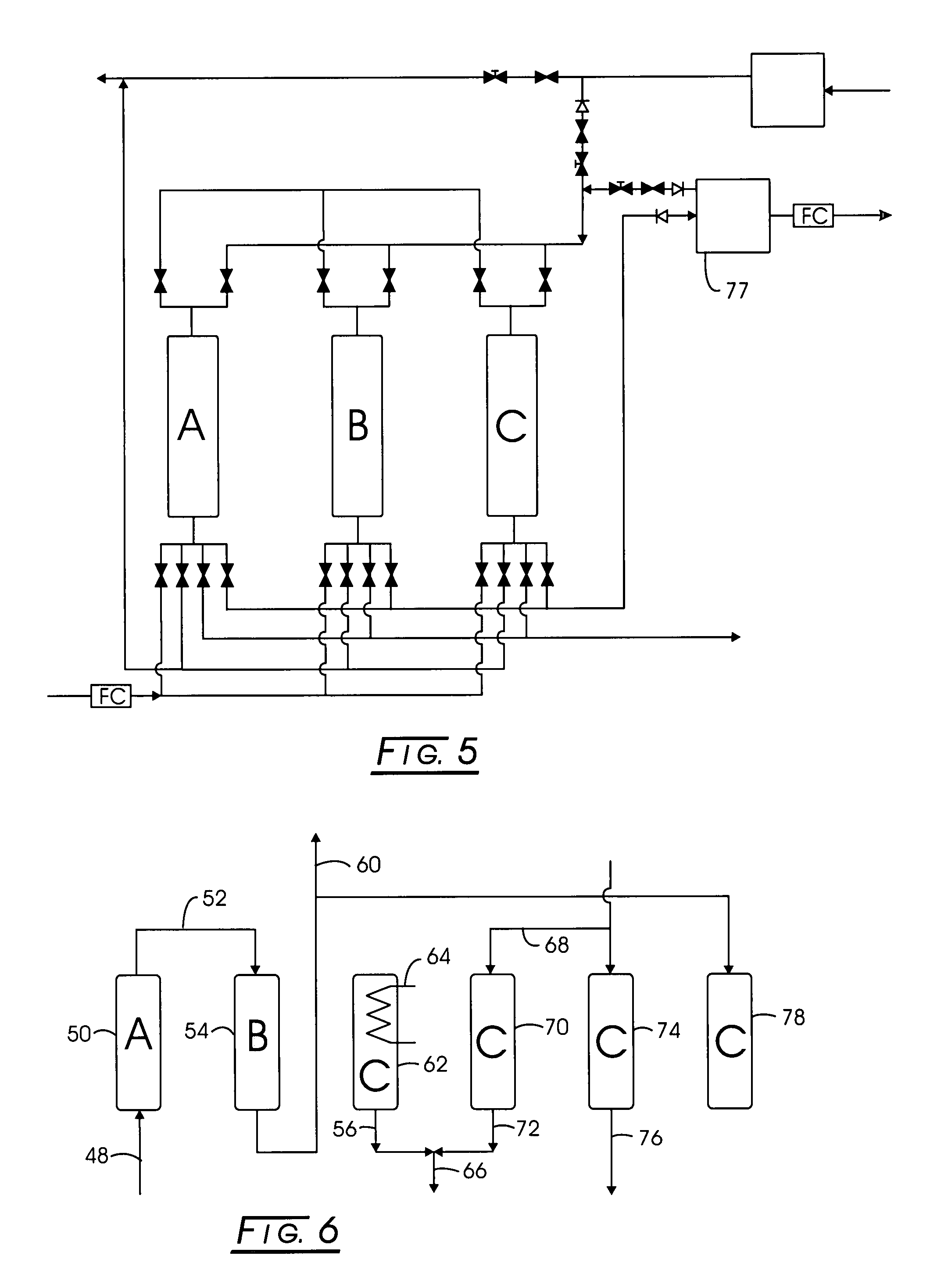
Multi-stage adsorption system for gas mixture sparation
a technology of adsorption system and gas mixture, which is applied in the direction of dispersed particle separation, separation process, waste based fuel, etc., can solve the problems of difficult to adapt that type of technology to produce lng economically on a small scale, different lng standards from pipeline standards, and significant differences in separation requirements, etc., to achieve the effect of less power and smaller vessels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0064]Unless explicitly stated otherwise, compositions that follow are listed in volumetric percentages, and the abbreviated unit ppm refers to parts-per-million by volume. Pressures are given in pounds per square inch, absolute, abbreviated as psia. Flow rates are listed as standard cubic feet per minute, abbreviated scfm, in this case 1 pound mole is equivalent to 379.4 standard cubic feet (scf).
first embodiment
[0065]Stage 1: Methane-bearing feed containing about: 52% methane, 34% carbon dioxide, 12% nitrogen, 2% oxygen, and 0.1% water vapor was separated using silica gel as the adsorbent in Stage 1. The maximum pressure, PH, was about 70 psia, the minimum pressure, PL, was about 0.8 psia, and the intermediate pressure, PA, was about 16 psia, while the intermediate pressure, PM, was about 35 psia. The effluent composition of the purified product was about 74% methane, 0.3% carbon dioxide, 23% nitrogen, 3% oxygen, and 0% water vapor. The corresponding time for each of the step is subject to optimization, and depends on the adsorbent, operating conditions, and vessel dimensions. Examples for this case are: 330 seconds for feed, 30 seconds for pressure equalization, 30 seconds for countercurrent blowdown, 160 seconds for evacuation, and 110 seconds for purge, 30 seconds for pressure equalization, and 300 seconds for pressurization. The net feed flow rate, per unit mass of adsorbent, was 0.059...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling points | aaaaa | aaaaa |
| dew point temperature | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com