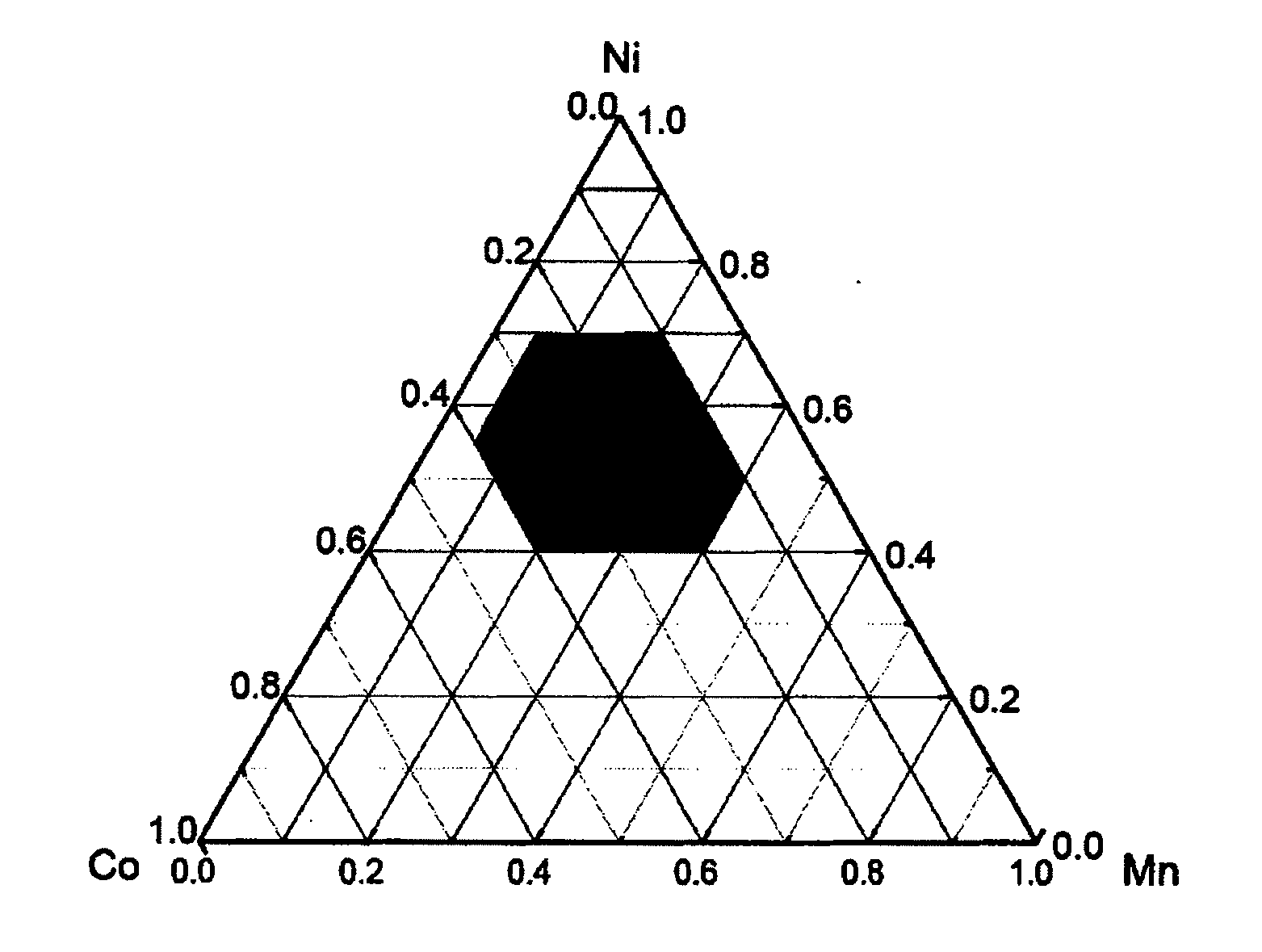
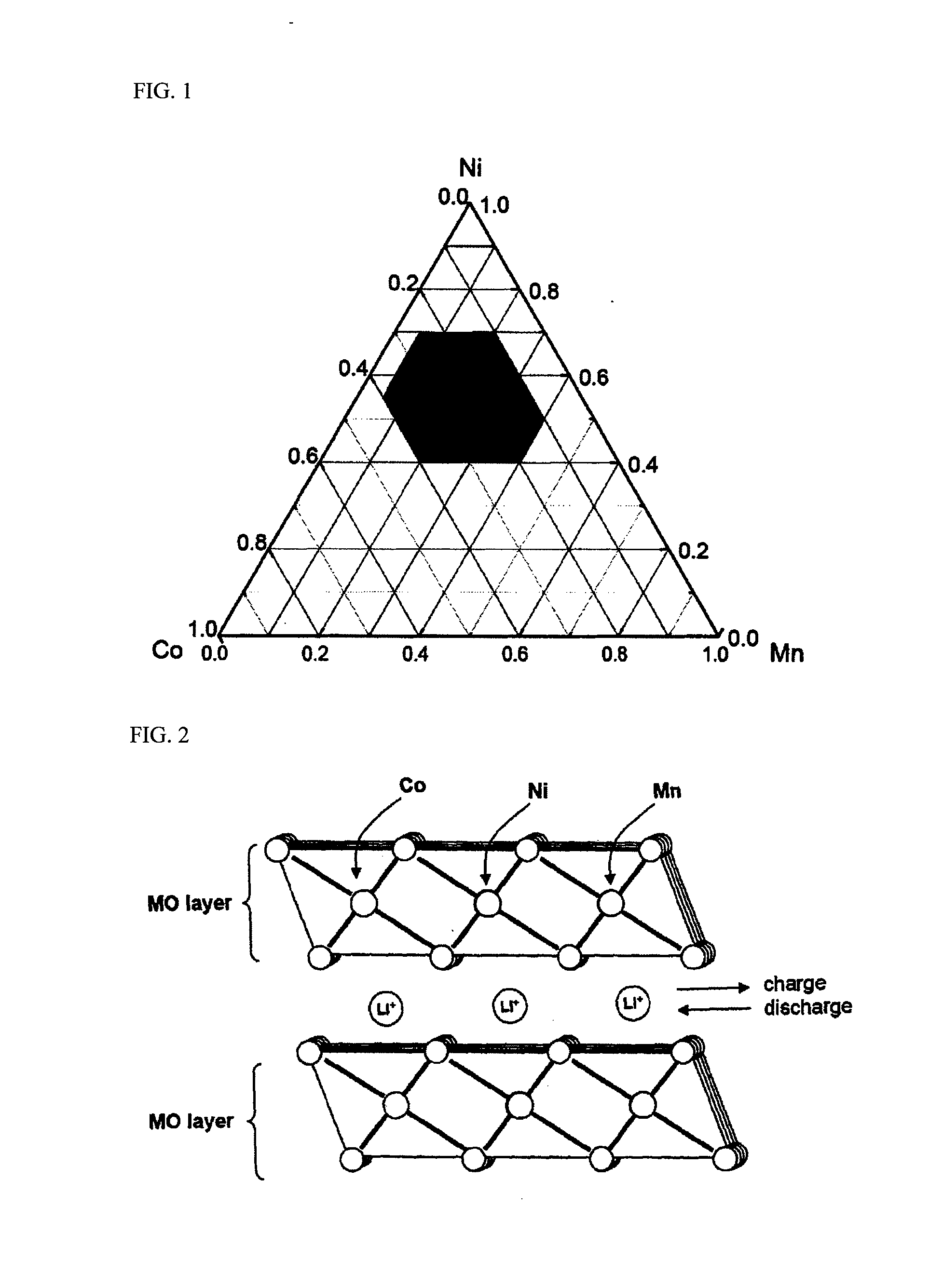
Cathode materials having high energy density and lithium secondary battery containing the same
a lithium secondary battery and cathode material technology, applied in the direction of non-metal conductors, cell components, oxide conductors, etc., can solve the problems of disadvantage, low energy density, and low energy density of lithium secondary batteries, so as to improve the mobility of lithium ions and rate properties, reduce the amount of transition metals present, and stabilize the crystal structure of cathode materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0109]LiCoO2 having a monolithic structure and D50 of about 15 to about 20 μm and LiNi0.53Co0.2Mn0.27O2 having D50 of about 5 to 8 μm, as an agglomerate of micro particles a size of about 1 to about 2 μm obtained in Preparation Example 1-1 were mixed at a ratio of 50:50 to prepare a cathode material mix.
[0110]The cathode material mix, Super P as a conductive material and polyvinylidene fluoride as a binder were mixed at a weight ratio 92:4:4, and N-methyl pyrrolidone (NMP) was added thereto to prepare a slurry. The cathode slurry was applied to an aluminum collector, followed by drying in a vacuum oven at 120° C. to produce a cathode.
[0111]In addition, mesocarbon microbead (MCMB) as an anode active material, super P as a conductive material and PVdF as a binder were mixed at a weight ratio of 92:2:6, followed by dispersion in NMP and coating on a copper foil, to produce an anode.
[0112]A porous membrane made of polypropylene was inserted between the anode and cathode thus obtained to...
example 2
[0113]A cathode material mix was prepared and a lithium secondary battery was produced in the same manner as in Example 1 except that a weight ratio of LiCoO2 and LiNi0.53Co0.2Mn0.27O2 in the cathode material mix was 70:30.
experimental example 1
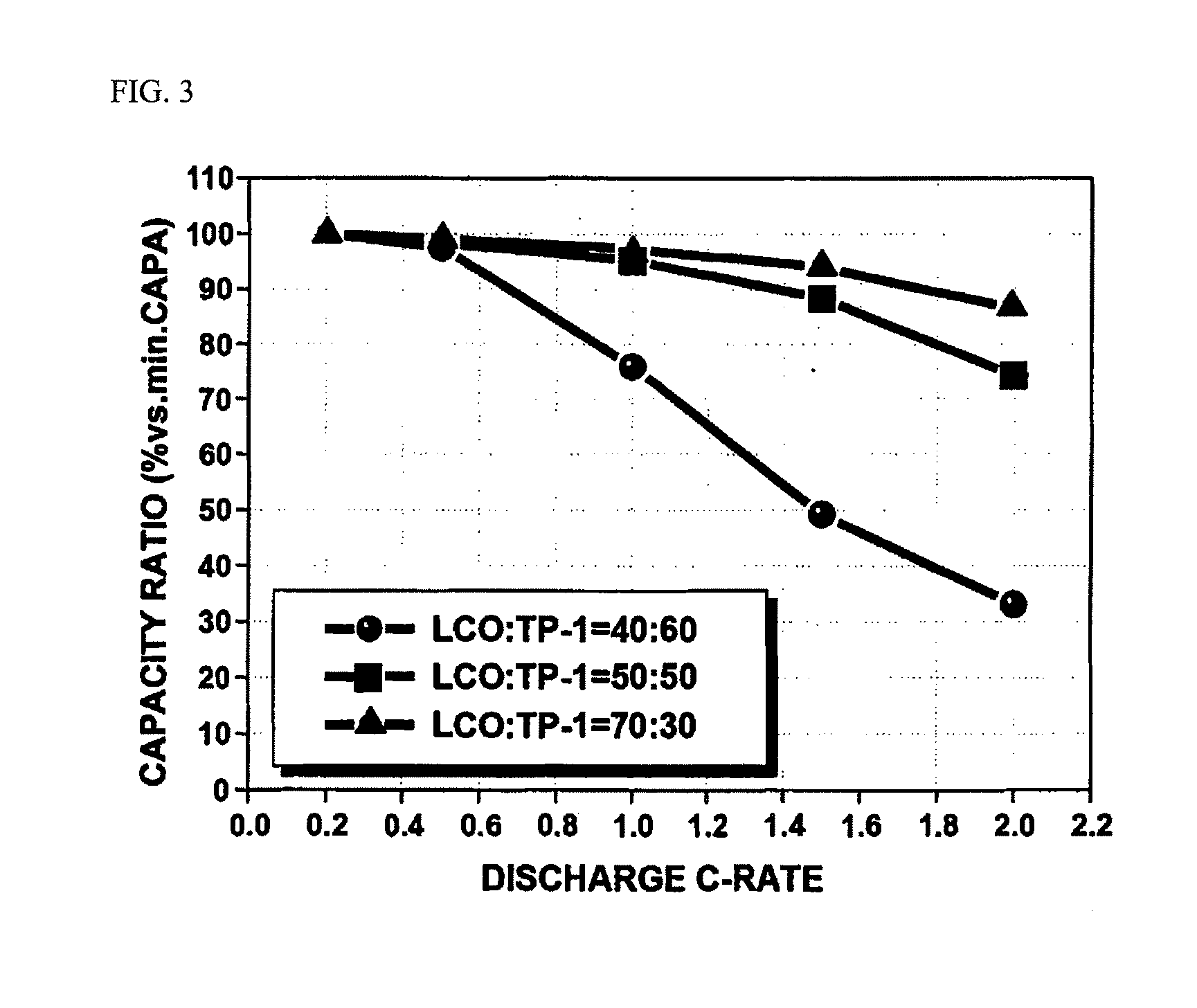
[0116]Discharge capacity (1C rate charge) of batteries produced in Examples 1 and 2 and the battery produced in Comparative Example 1 were measured at 0.2C, 0.5 C, 1C, 1.5C and 2C rate and a ratio of discharge capacity at each C-rate with respect to 5C rate capacity was calculated. The results thus obtained are shown in FIG. 3.
[0117]As can be seen from FIG. 3, discharge capacity of the battery of Comparative Example 2 rapidly decreases, as C-rate increases, and on the other hand, batteries of Examples 1 and 2 of the present invention exhibited considerably superior C-rate properties, and in particular, the battery of Example 2 containing 30% of oxide (b) exhibited superior C-rate properties in which discharge capacity is as high as 90% or more at a 2C rate. In addition, it can be seen that this improvement in C-rate properties was exhibited even at a low C-rate of 1C, and batteries of Examples 1 and 2 exhibited more considerable improvement in discharge properties, as C-rate thereof...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| total weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com