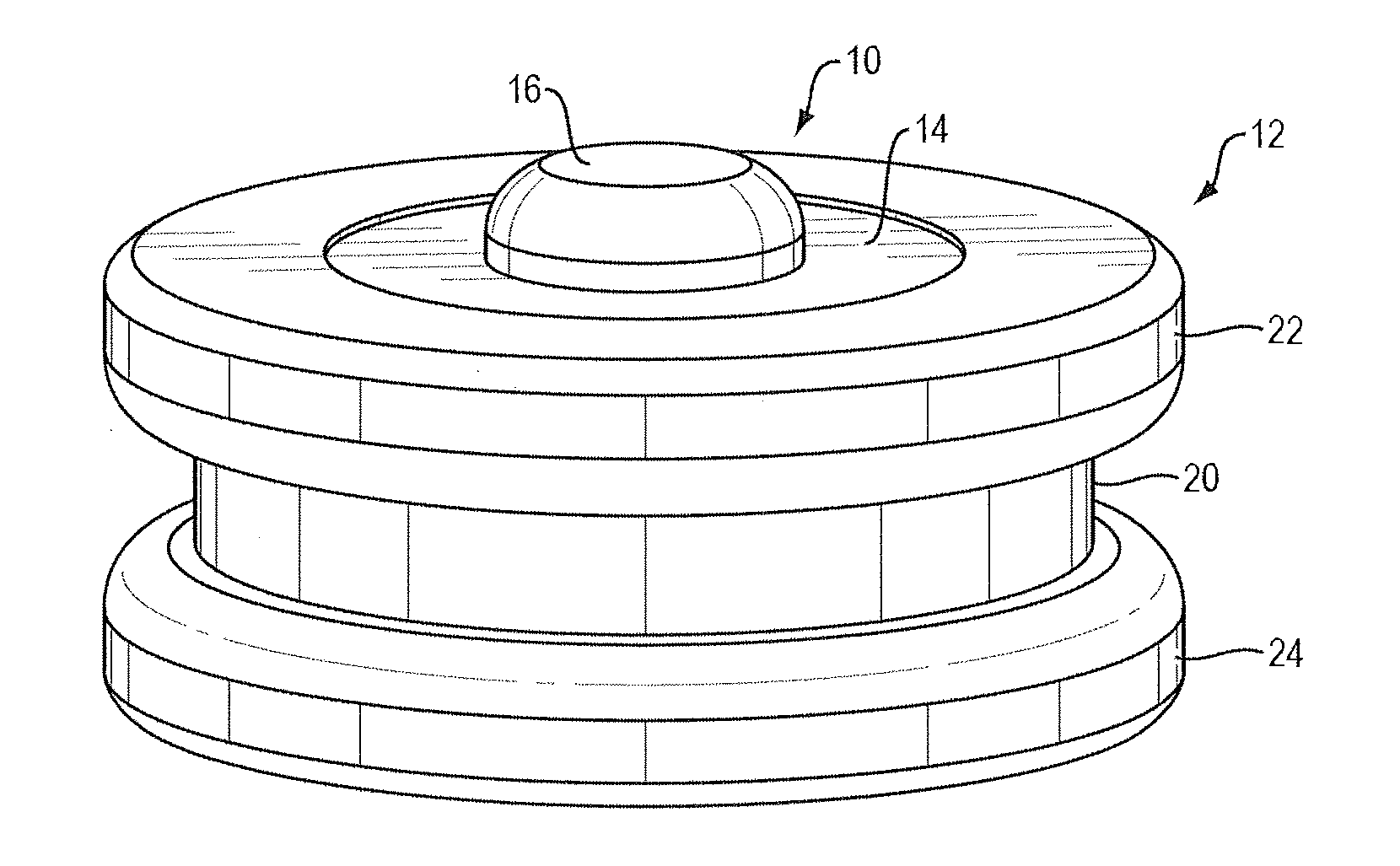
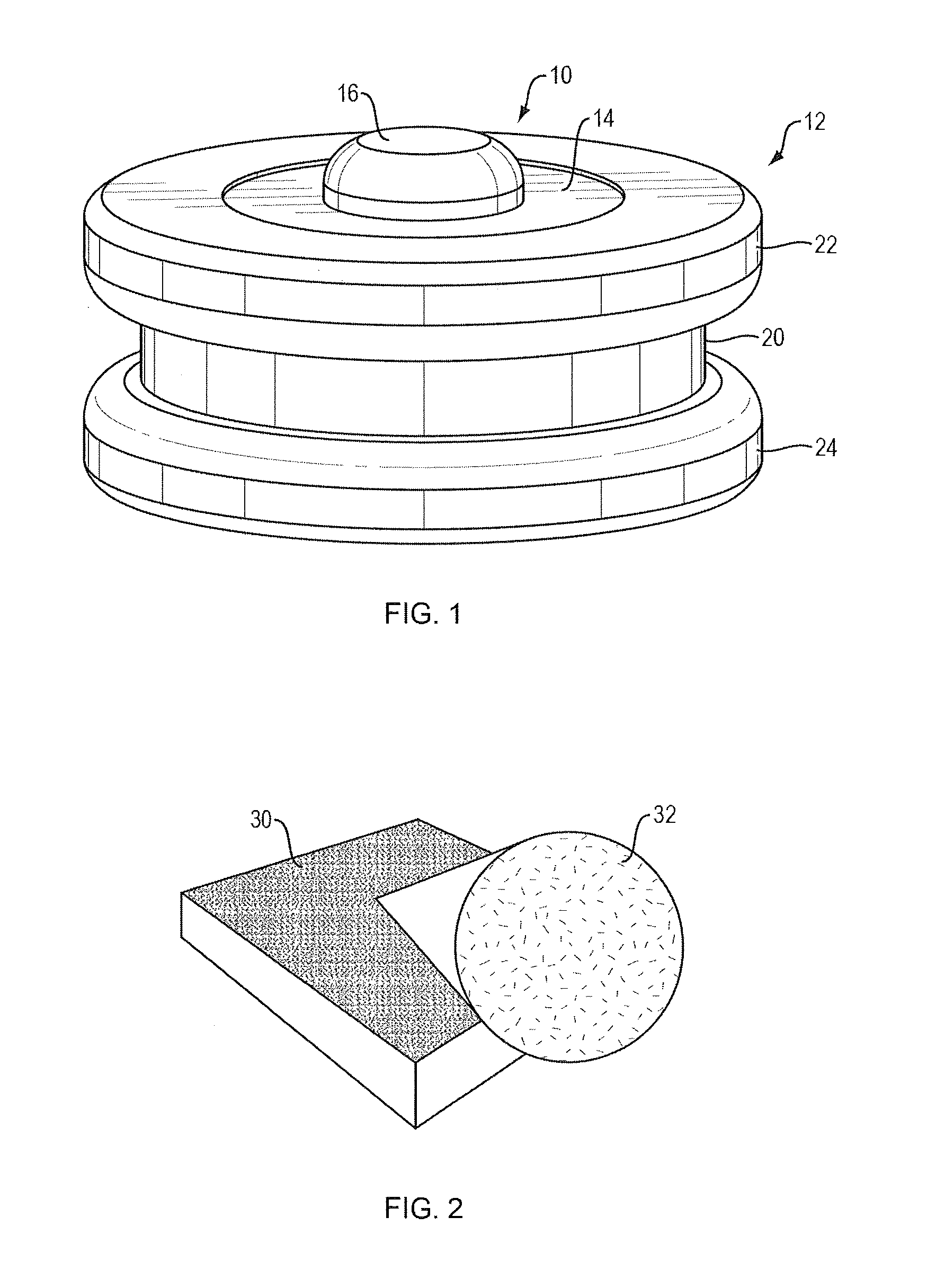
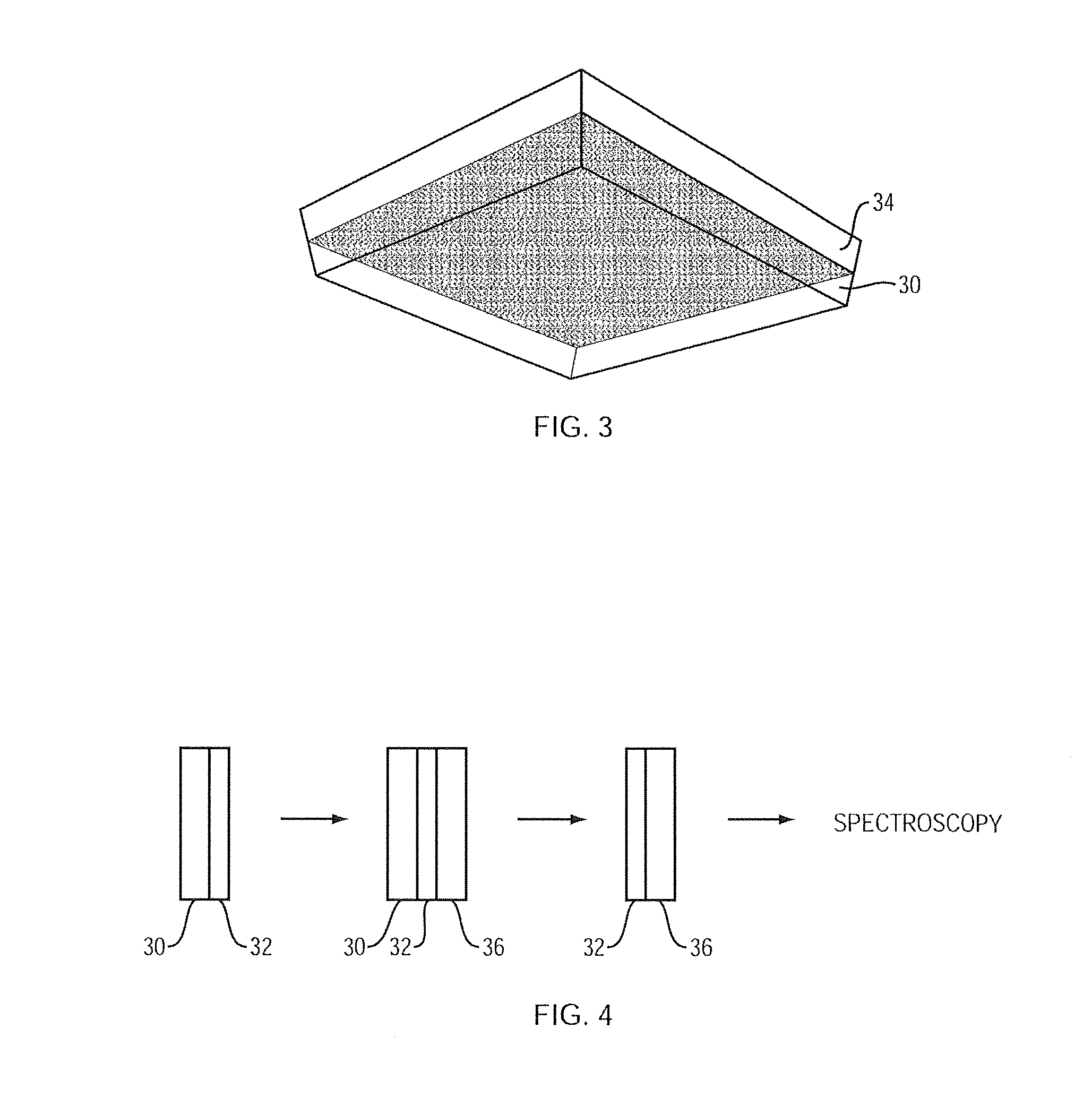
Apparatus and method for preconcentrating and transferring analytes from surfaces and measurement thereof using spectroscopy
a technology of surface analytes and spectroscopy, applied in the direction of instruments, separation processes, laboratory glassware, etc., can solve the problems of compromising mission operations, compromising positive identification, and major challenge in field measurement of suspected hazardous chemicals, so as to facilitate efficient and/or selective transfer of analytes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Silver Cyanide Detection in an Aqueous Residue on a Solid Surface Using Transmission and ATR Infrared Spectroscopy
[0055]In this example, an aqueous residue containing toxic silver cyanide was collected onto the collection substrate of the present invention and analyzed in 3 different ways using infrared spectroscopy to demonstrate several embodiments of the invention. The collection substrate was acquired from Orono Spectral Solutions, Inc. (OSS) of Bangor, Me. As used herein, “cyanide” refers to any chemical compound including a Carbon atom triple-bonded to a Nitrogen atom. It may be referred to herein from time to time as “CN.” Silver Cyanide may be referred to herein as AgCN.
[0056]Preparation of the AgCN Residue
[0057]AgCN residue was prepared by dissolving 100 mg of silver nitrate into 1 mL of deionized water. Once dissolved, 25 mg of sodium cyanide was added to the solution, which then turned cloudy as silver cyanide particles were formed. This slurry was then poured onto a labo...
example 2
Silver Phosphate Detection in an Aqueous Residue on a Solid Surface Using ATR Infrared Spectroscopy
[0068]In this example, an aqueous residue containing silver phosphate was collected onto the collection substrate of the present invention and analyzed in two different ways using infrared spectroscopy to demonstrate several embodiments of the invention. The collection substrate was acquired from OSS.
[0069]Preparation of the Silver Phosphate Residue
[0070]Silver phosphate residue was prepared by dissolving 200 mg of silver nitrate into 1 mL of deionized water. Once dissolved, 25 mg of sodium cyanide was added to the solution, which then turned cloudy as silver phosphate particles were formed. This slurry was then poured onto a laboratory bench, to generate an aqueous residue.
[0071]Collection of Silver Phosphate Residue Using OSS Collection Substrate
[0072]The residue was collected using OSS Collection Substrate (PN 092385) and a wiping motion to collect the residue. For this example, two...
example 3
Detection of Residue Containing BG Spores on Surfaces
[0079]In this example, a residue containing Bacillus globigii spores (BG) was collected onto the collection substrate of the present invention and analyzed using ATR infrared spectroscopy. The collection substrate was acquired from OSS.
[0080]Preparation of the BG Spore Residue
[0081]BG spore residue was prepared by applying a fine powder of BG spores on a steel surface.
[0082]Collection of BG Spore Residue Using OSS Collection Substrate
[0083]The residue was collected using OSS Collection Substrate (PN 04031982) and a wiping motion to collect the residue.
[0084]ATR FTIR Spectroscopy of OSS Collection Substrate
[0085]ATR FTIR spectroscopy (using a Bruker Alpha spectrometer outfitted with a Bruker Platinum ATR accessory) was used to measure BG spore residue collected on the OSS Collection Substrate (FIG. 10). The peaks near 1640 and 1560 cm−1 are due to amide bands from protein material in the spore. The peak near 1100 cm−1 is due to pol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Transparency | aaaaa | aaaaa |
| Hydrophobicity | aaaaa | aaaaa |
| Affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com