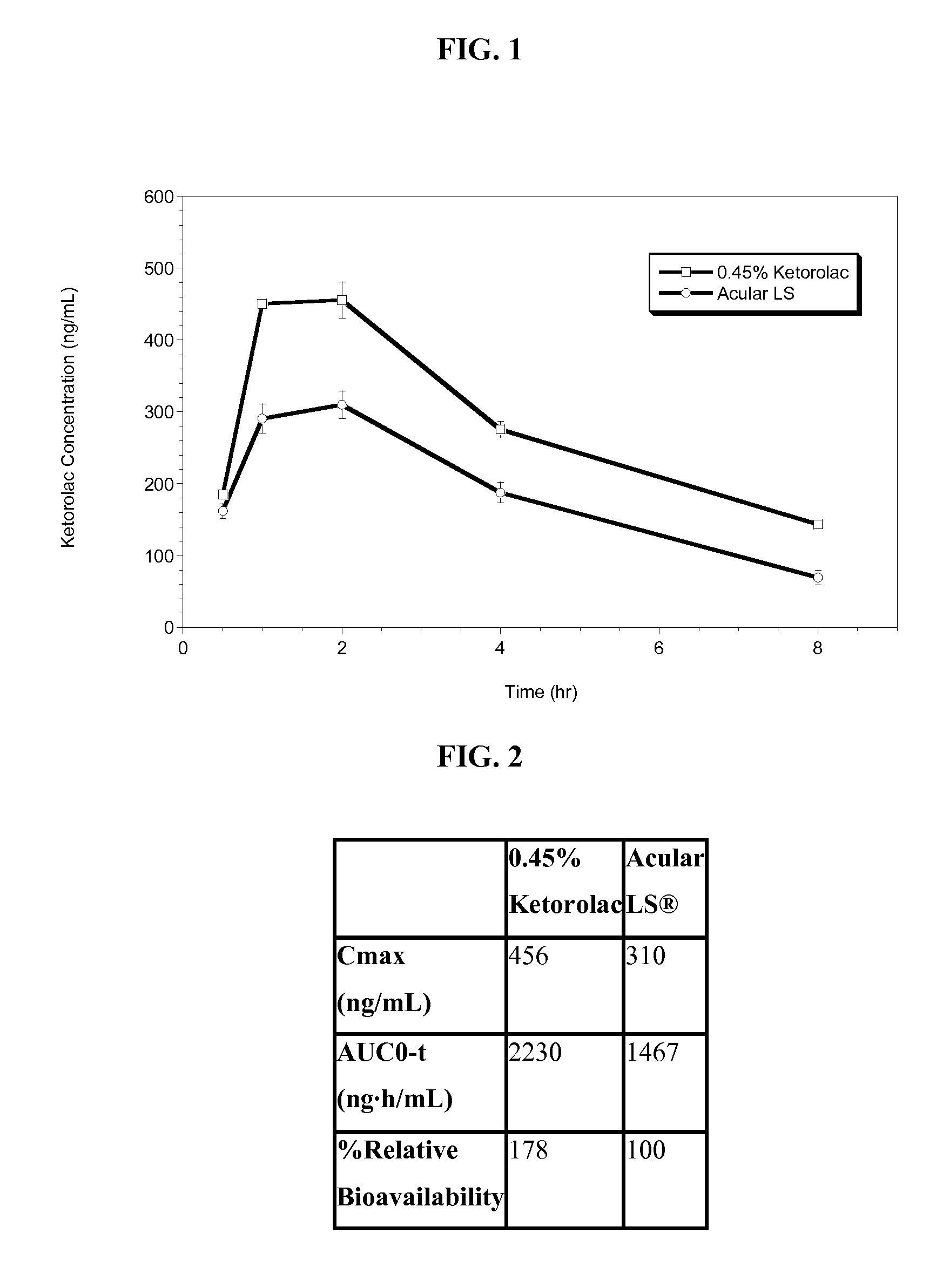
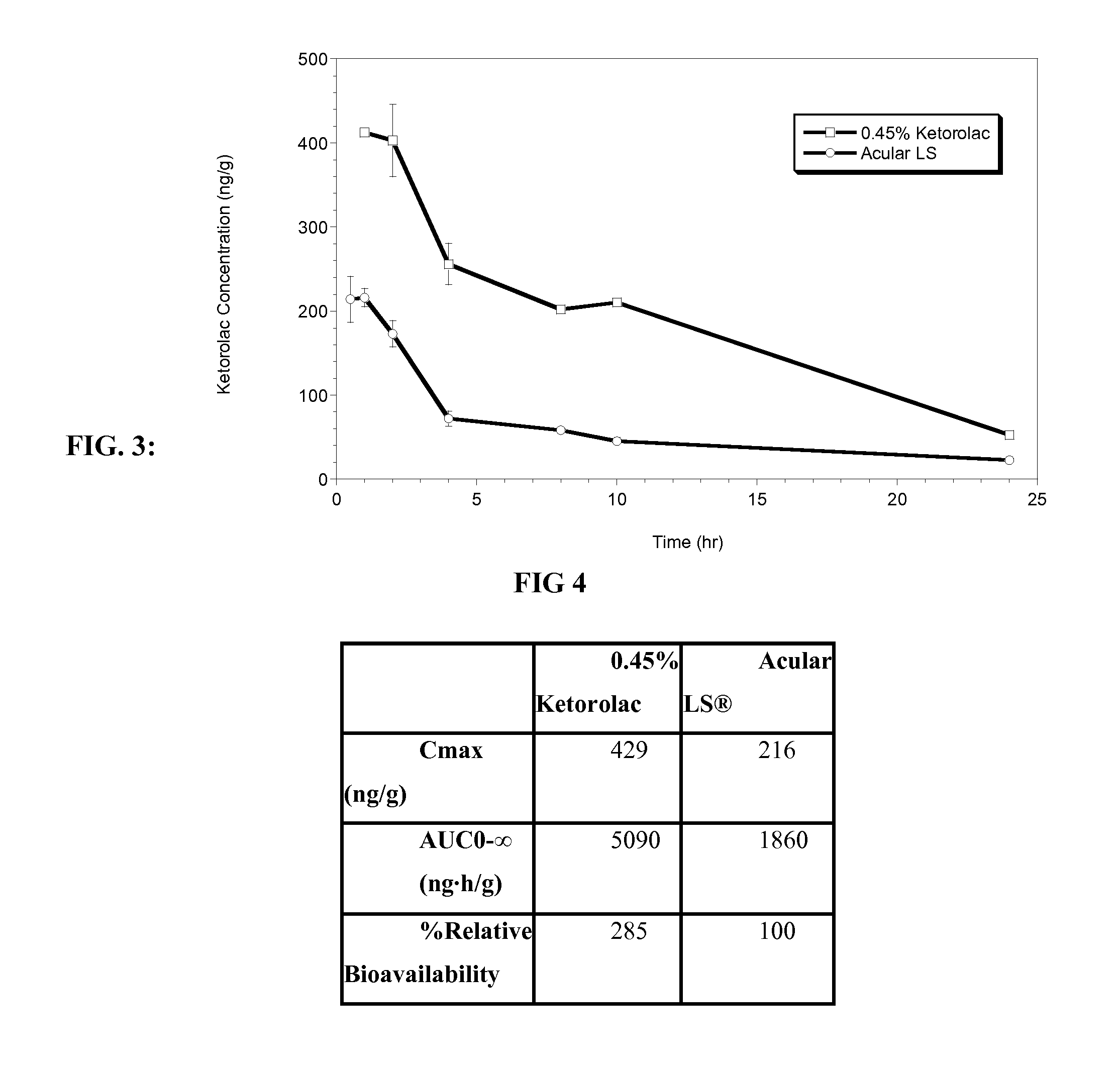
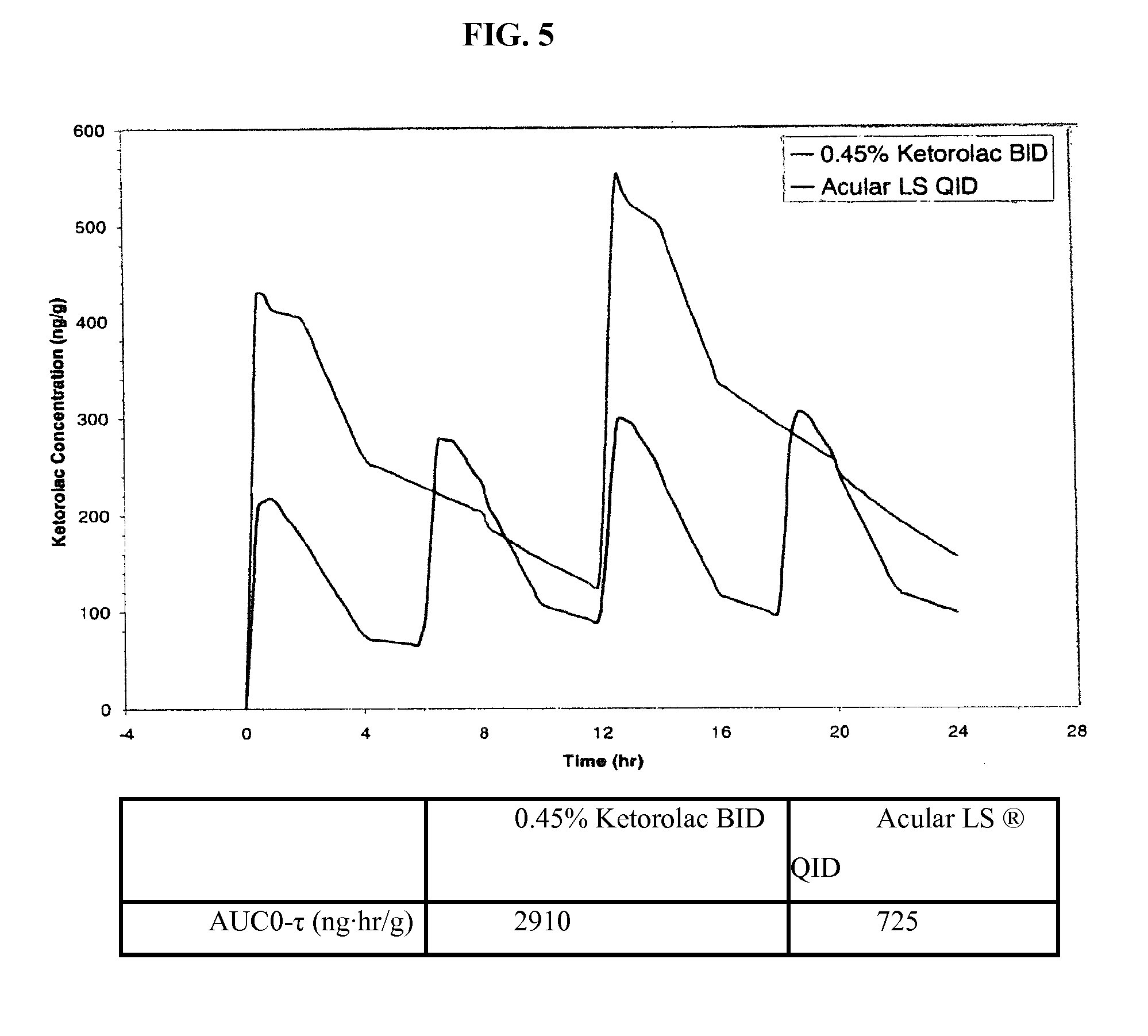
Ketorolac tromethamine compositions for treating or preventing ocular pain
a technology of ketorolac and compositions, applied in the field of pharmaceutical compositions, can solve the problems of ocular irritation, 5% ketorolac formulation is ocular irritation, primarily burning and stinging, and is associated with some adverse effects, so as to reduce the required daily dosage and prolong the effect of ketorola
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0041]Unless otherwise specified, all steps in this procedure were carried out at room temperature. The following procedure was followed in accordance with the amounts listed in Table 1 below. Purified water was charged into the main batch vessel. Mixing was initiated to produce a vortex sufficient to disperse and / or dissolve all product ingredients without excessive aeration or foam formation. The following components were added directly into the vortex in order, allowing each to dissolve before adding the next: sodium chloride, calcium chloride, dihydrate magnesium chloride, hexahydrate, boric acid, sodium borate, sodium carboxymethyl cellulose as a an percent aqueous solution comprising including a mixture of 65% medium molecular weight and 35% high molecular weight carboxymethyl cellulose. The solution was mixed for no longer than 15 minutes. A specified amount of 1N sodium hydroxide, was then added. The pH was checked and, if needed, was adjusted to 7.3 with 1N sodium hydroxide...
example 2
[0042]Unless otherwise specified, all steps in this procedure were carried out at room temperature. The following procedure was followed in accordance with the amounts listed in Table 2 below. Purified water at 90% of batch size was charged into the main batch vessel. Mixing was initiated to produce a vortex sufficient to disperse and / or dissolve all product ingredients without excessive aeration or foam formation. The following components were added directly into the vortex in order, allowing each to dissolve before adding the next: sodium chloride, edetate disodium, octoxynol-40 (as a 70% stock solution) and benzalkoniurn chloride (as a 10% stock solution). The amount of benzalkonium chloride added took into account the assay of the stock solution used. The solution was mixed for no longer than 15 minutes. A specified amount of 1N sodium hydroxide, 1.85 mL per liter of final bulk product, was then added. The pH was checked and if needed was adjusted to 10.7-11.0 with 1N sodium hyd...
example 3
[0043]This example was prepared according to the procedure of Example 1, except that hydroxypropyl cellulose was used in place of the sodium carboxymethyl cellulose in an amount sufficient to provide a viscosity equivalent to the viscosity of the composition of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com