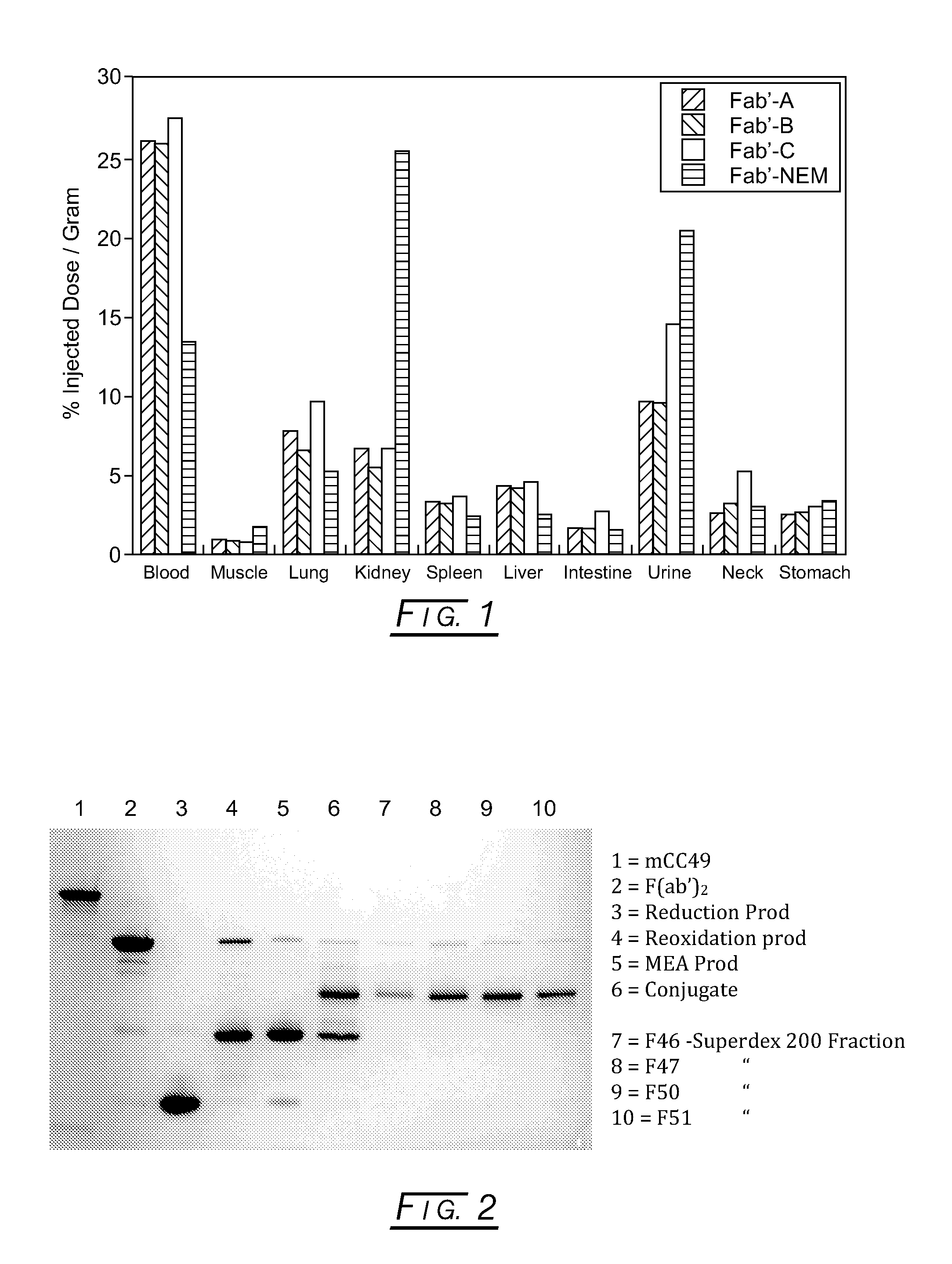
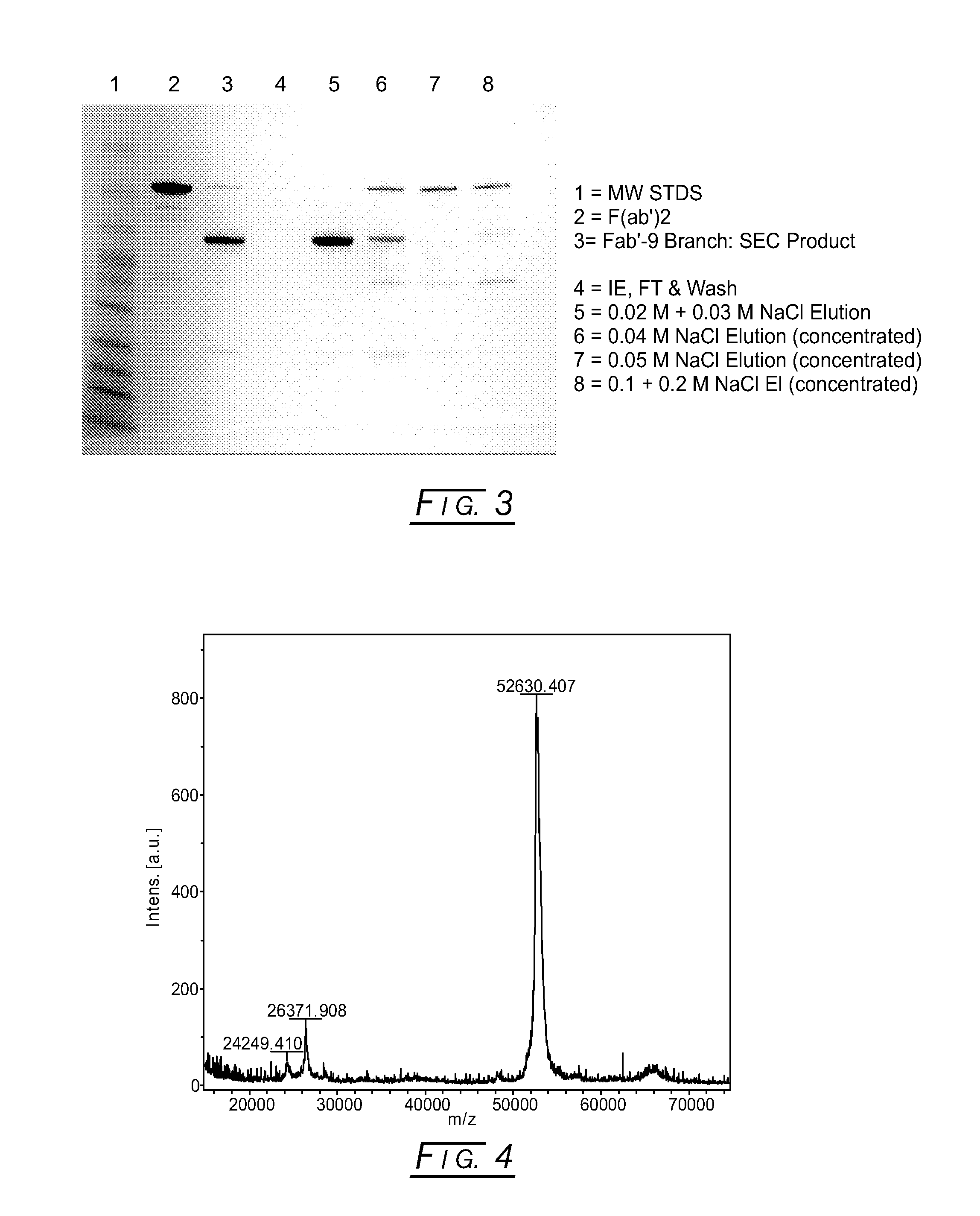
Branched Discreet PEG Constructs
a discrete, polyethylene glycol technology, applied in the direction of peptides, medical preparations, peptide/protein ingredients, etc., can solve the problems of toxicological or immunological tolerance, potential customers often will not even consider purchasing pegylation reagents, and drawbacks of these polymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Basic Branched Core, Branched dPEG Constructs and Hetero, BC1, BC2
[0305]
Preparation of Maleimide Derivatives MAL-dPEG4-Tris(m-dPEG24)3; MAL-dPEG4-Tris(-dPEG24 acid)3; MAL-dPEG4-Tris(-dPEG4-Tris(m-dPEG12)3)3
[0306]To a solution of each of the amines (0.5 g) dissolved in glacial HOAc (5 mL) was added maleimidopropionate N-hydroxysuccinimide ester (2 equiv.). That solution was stirred and heated under microwave at 150° C. for 30 min. Upon completion of reaction, 5 mL of water was added. The crude reaction solution (10+ mL) was purified on a Biotage C18 FLASH (25+ M) column via a gradient elution. The gradient mixture was composed of MeOH and 0.1% aqueous HOAc (pH 3.25). Starting with 25% MeOH / 75% 1% HOAc solution. The initial solvent mixture was held for 2 min, increased to 100% MeOH over the next 15 min, and then held at 100% MeOH for 8 min). Isolated yields of the purified and respective MAL-branched dPEGs were in the range 67-91%.
PhthN-dPEG4-Tris(TFP)3:
[0307]
A solution of Phth-dPEG4...
example 2
Preparation of Exendin Conjugates
Materials
[0329]Exendin 4-Cys (Exe-4) was synthesized by Ohio Peptide using solid phase peptide synthesis techniques and purified by reverse phase HPLC. Exe-C contains the 39 amino acids of native exendin 4, a Gila monster salivary protein, attached to a C-terminal cysteine.
Conjugation
[0330]The following conjugates were prepared:
1. Exe-4-S-N-Ethyl Malimide
2. Exe-4-S-MAL-m-d PEG-12
3. Exe-4-S-MAL-dPEG12-Tris(m-dPEG-24)3
[0331]4. (Exe-4-S-MAL-d PEG-2)2-d PEG-Lysine-dPEG-4-amido-d PEG-12-Tris(m-dPEG-24)3
[0332]The conjugation reactions for Compounds 2-4 (see above) contained 1.6 mL of 1.25 mg / mL Exe-4 in deionized water, 0.2 mL of a 1 M sodium phosphate buffer (pH 6.5) containing 10 mM EDTA, and 0.2 mL of a 20 mM solution of the maleimide compound dissolved in dimethylacetamide. After incubation for 1 hour at room temperature, 0.2 mL of a 100 mM solution of N-ethylemaleimide was added to each reaction. The reactions were allowed to proceed for an addition...
example 3
Attachment Core Ac and Template Related Examples
PhthN-dPEG4-Tyr-OBn
[0335]
A 1 L 3-neck RBF fitted with nitrogen blanket and cooling bath was set up for this reaction. PhthN-4-acid (17.6 g, 44.5 mmol) was dissolved in the flask followed by N-methyl morpholine (14.41 ml, 142 mmol) and H2N-Tyr(OH)—OBn toluene sulfonate (21.72 g, 49.0 mmol). HOBt hydrate (1.128 g, 6.68 mmol) was added in a single portion and the mixture was stirred for 10 minutes and then cooled in an ice bath. EDC (12.80 g, 66.8 mmol) was added in a single portion and the reaction was slowly allowed to warm to room temp overnight. HPLC (Acid4020 method) indicated complete consumption of sm and formation of a less polar product. TLC (95:5 and 90:10 DCM:IPA) also indicated complete consumption of dPEG. The solvent was removed under reduced pressure and the residue was taken up in 300 mL H2O and extracted with EtOAc (3×300 mL). TLC indicated the product had been extracted. They were washed with 10% HCl (3×75 mL), sat aq Na...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com