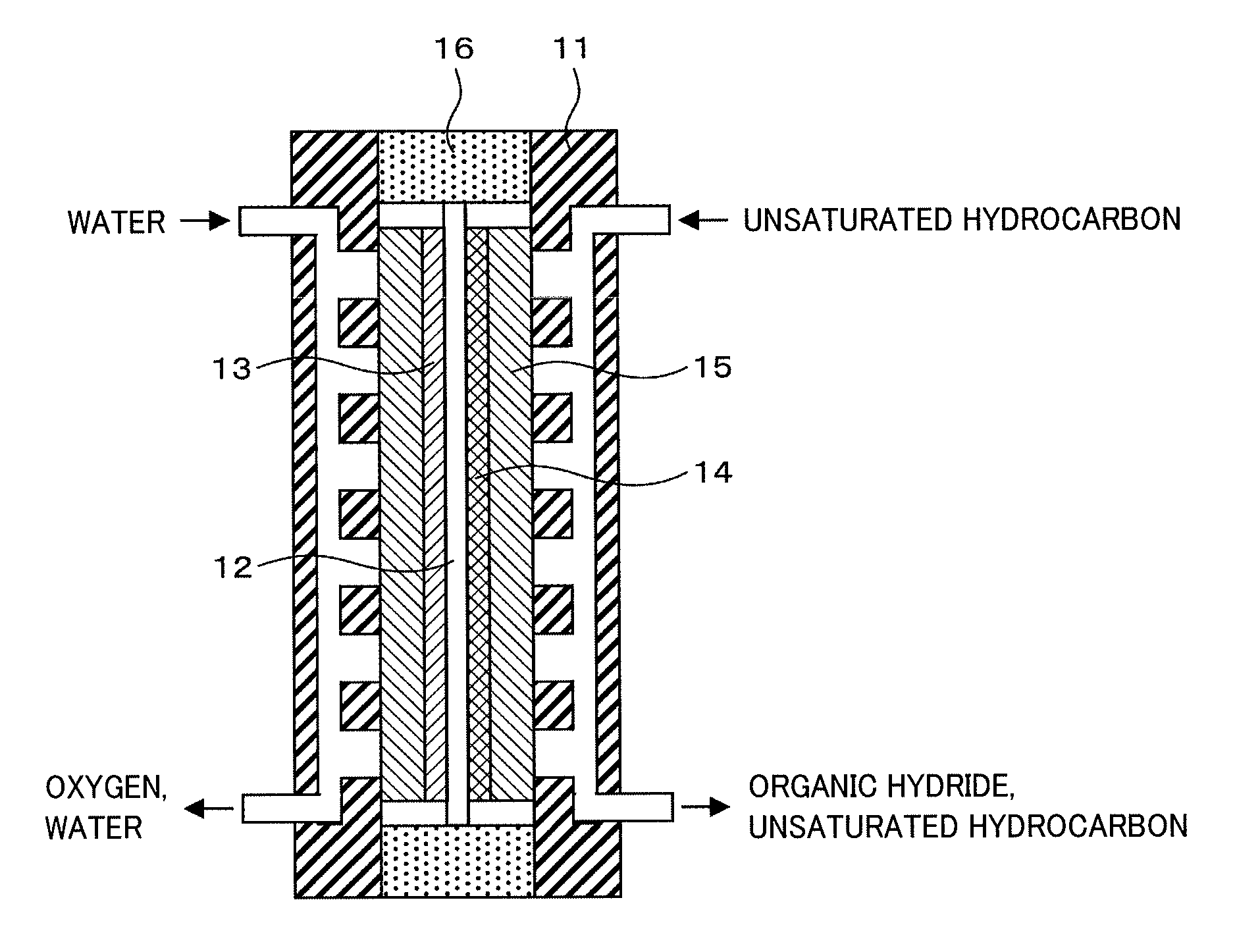
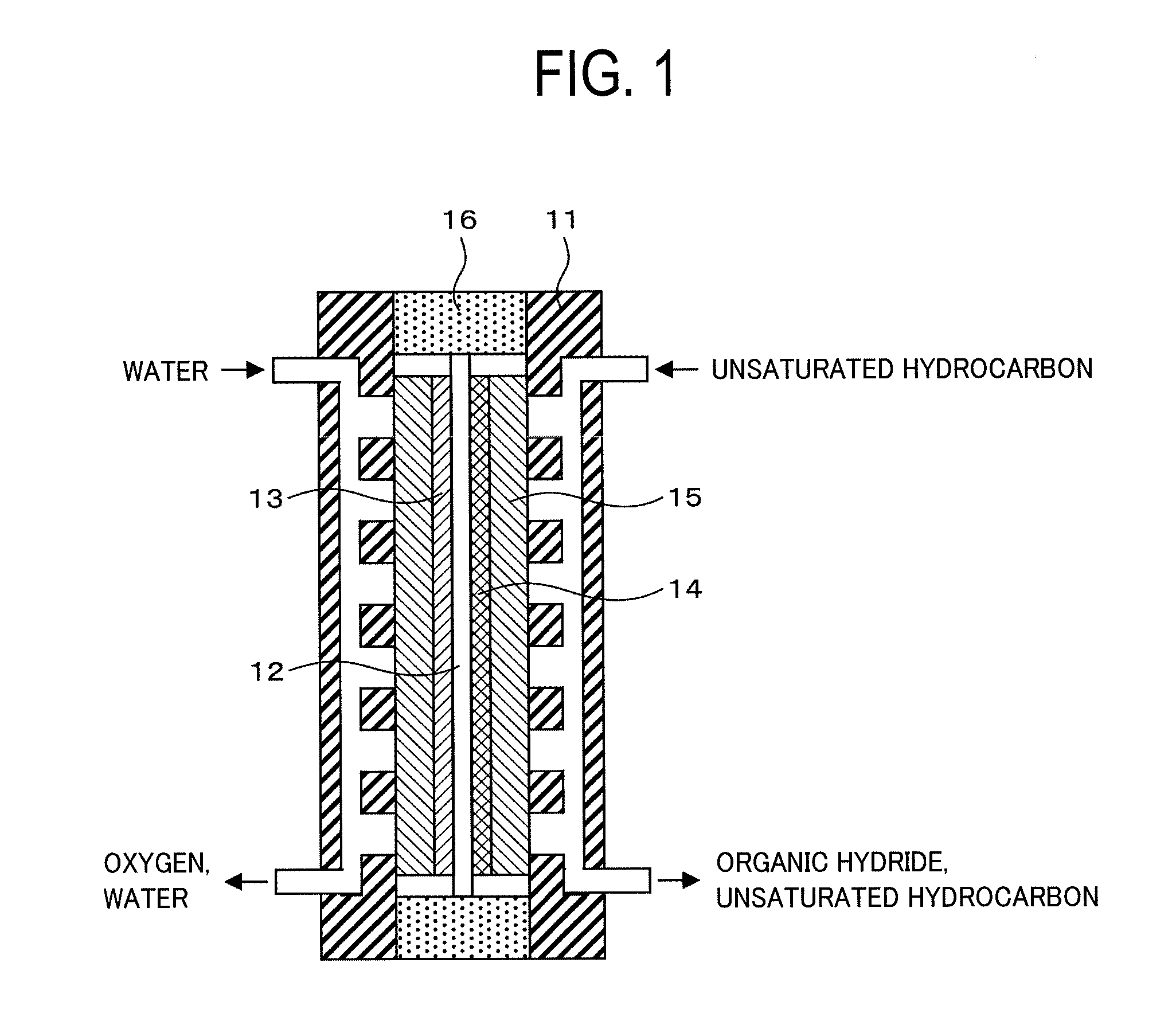
Membrane Electrode Assembly and Organic Hydride Manufacturing Device
a technology of organic hydride and membrane electrode, which is applied in the direction of electrolytic organic production, electrogenerative processes, electrolytic coatings, etc., can solve the problems of difficulty in obtaining higher energy efficiency, and achieve the effect of reducing the wettability of unsaturated hydrocarbons and high energy efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first example
[0051]As a catalyst, a catalyst where 30 wt % of Pt particulates was dispersed and supported on carbon black was used. Firstly, 100 g of this catalyst was preheated for one hour at 105° C. Subsequently, sulfur trioxide heated to 100° C. was transferred at 12 vol. % of concentration to dry air, and reacted with the catalyst. Reaction time was two hours. Subsequently, it was cooled, the catalyst was submitted to ion exchanged water, stirred and filtrated, and washed with ion exchanged water until pH of the filtrate became constant.
[0052]When the infrared absorption spectrum was measured to the obtained catalyst, the peaks were observed at 620 cm−1, 1037 cm−1 and 1225 cm−1. This was considered as the peak based on the sulfonate group —SO3H, and it was confirmed that the sulfonate group was introduced on the surface of the carbon black which was a carrier. The equivalent of the introduced sulfonate group was 1.8 milliequivalent / g dry carbon carrier.
[0053]The above-obtained catalyst was ...
second embodiment
[0062]As a catalyst, a catalyst where 30% (wt) of Pt particulates was dispersed and supported on carbon black was used. 10 g of this catalyst and 15 g of anhydrous aluminum chloride (AlCl3) were mixed and thiophosphoryl chloride (PSCl3) was added gradually. The temperature of PSCl3 was fixed at 35° C. and 54 mL was added slowly. Subsequently, it was kept at 75° C. for 45 minutes. After cooling, 50 mL of chloroform was added and filtrated. After washing with diethyl ether thoroughly, 200 mL of ion-exchanged water was added and refluxed for 20 hours. When the infrared absorption spectrum of the obtained catalyst was measured, the peaks were observed at 1000 to 1120 cm−1 and 840 to 910 cm−1. This was considered as the peak based on phosphonate, and it was confirmed that phosphonate groups were introduced onto the surface of the carrier carbon black. The equivalent of the introduced phosphonate groups was 1.8 milliequivalent / g dry carbon carrier.
[0063]The prepared catalyst was used for ...
third example
[0066]As a catalyst, a catalyst where 30 wt % of Pt particulates was dispersed and supported on carbon black was used. Oxygen plasma was irradiated to this catalyst. The device to use for irradiation was a plasma device, Cat. No. PDC210 manufactured by Yamato Glass, and the pressure in the chamber before introducing oxygen was 0.1 Torr or lower, and the pressure after introducing oxygen was 0.5 Torr. The output of a high frequency power source of the device was 100 W, and plasma irradiation time was 150 seconds. When infrared absorption spectrum of the yielded catalyst was measured, a broad peak was observed at 3000 to 3600 cm−1. This was considered as the peak based on the hydroxyl group —OH, and it was confirmed that the hydroxyl group was introduced on the surface of Pt supported carbon black.
[0067]The prepared catalyst was used for cathode catalyst to prepare an MEA. The preparation was conducted in the method and condition for the preparation similar to those of First Example.
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical conductor | aaaaa | aaaaa |
| Efficiency | aaaaa | aaaaa |
| Wettability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com