Ophthalmic compositions with improved dessication protection and retention
a technology of applied in the direction of drug compositions, biocides, inorganic non-active ingredients, etc., can solve the problems of cumbersome and time-consuming treatment, high cost, and inability to meet the needs of patients, so as to improve the desiccation protection and retention characteristics, the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0030]
Ingredient% W / VHydroxypropyl Guar0.025 to 0.8 Sodium Hyaluronate0.13 to 0.17Boric Acid0.35Sorbitol1.4PEG 4000.4EDTA Sodium0.025Propylene Glycol0.3Potassium Chloride0.12Sodium Chloride0.1Polyquaternium-10.001 + 10% excess2-Amino-2-methylpropanol0.27Sodium Hydroxide / Hydrochloric Acidq.s. pH 7.9Purified Waterq.s. 100%
example 2
[0031]Guar and hyaluronate compositions of the present invention were autoclaved under standard conditions. As shown below in Table 1, the composition comprising sorbitol has a stabilized molecular weight when compared with the composition that did not contain sorbitol.
TABLE 1[HA][HA][HA]Sodium HyaluronatePowderalonewith SorbitolInitial (Powder, 1 × 106 g / mol)1.7——(PD = 1.5)Molecular Weight Before—1.91.9autoclave (1 × 106 g / mol)(PD = 1.4)(PD = 1.5)Molecular Weight After Autoclave—0.41.4(1 × 106 g / mol)(PD = 1.6)(PD = 1.3)pH before Autoclave—7.07.0pH after Autoclave—6.56.8Viscosity at 0.1 s−1 Before—241249AutoclaveViscosity at 10 s−1 After Autoclave—2496
example 3
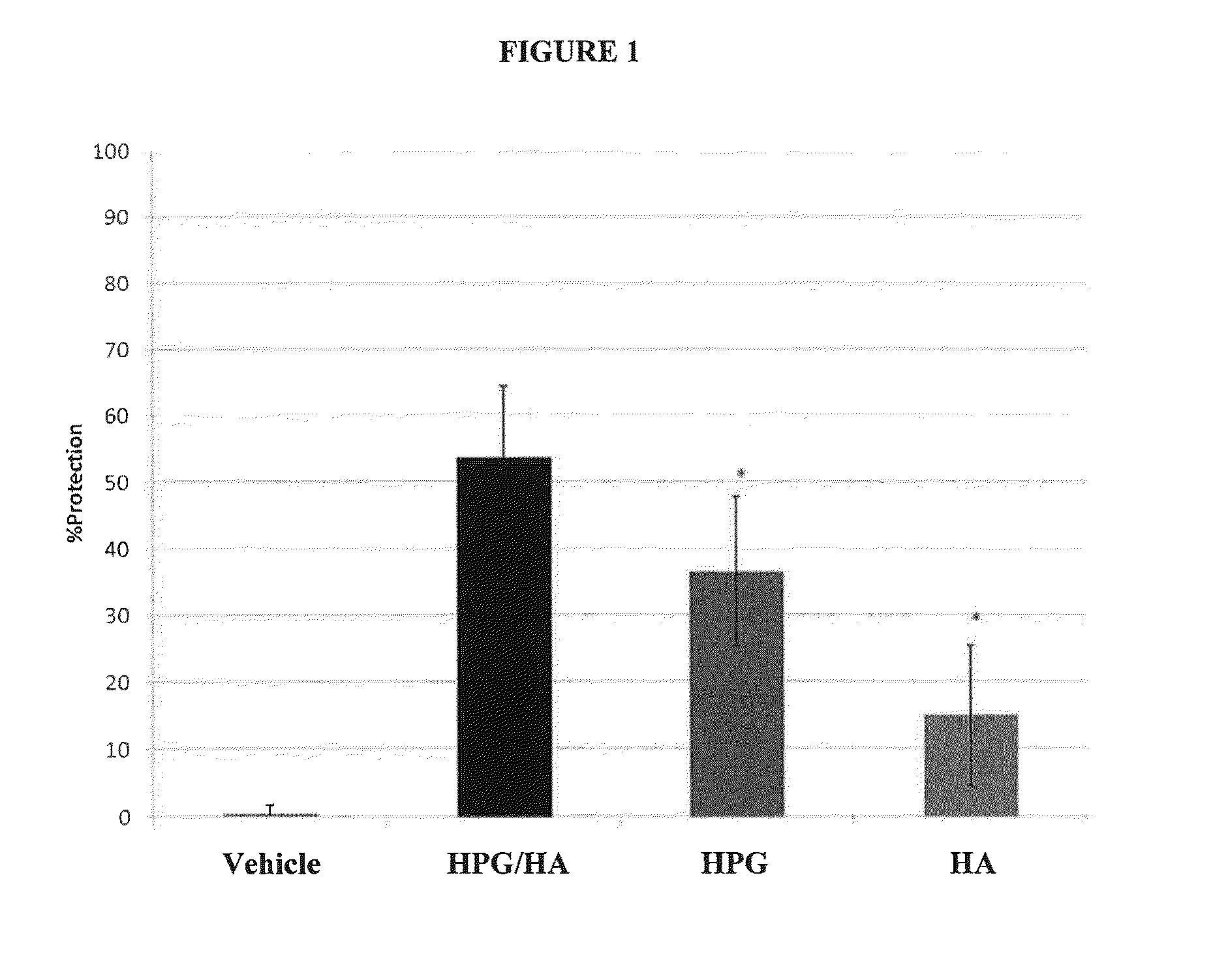
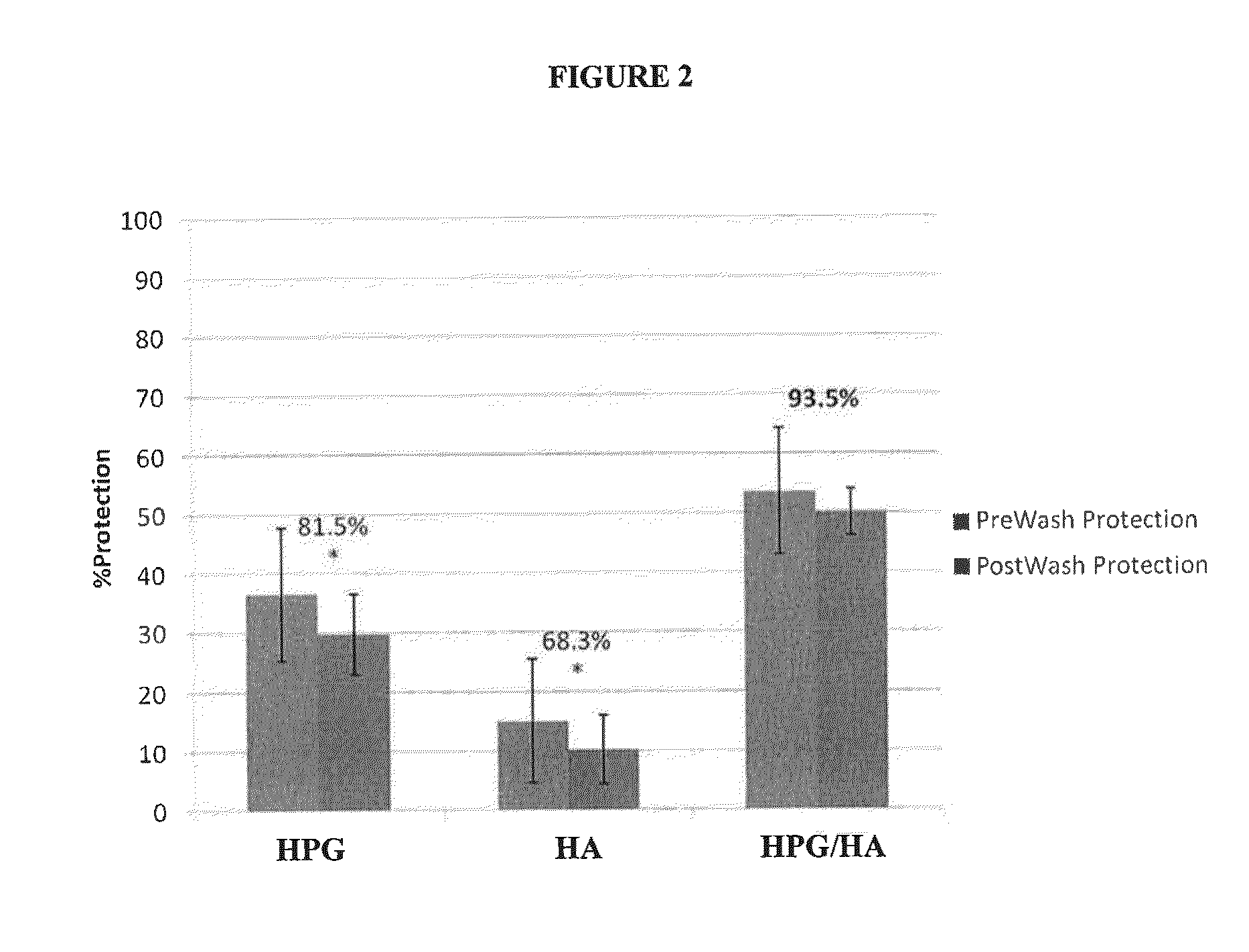
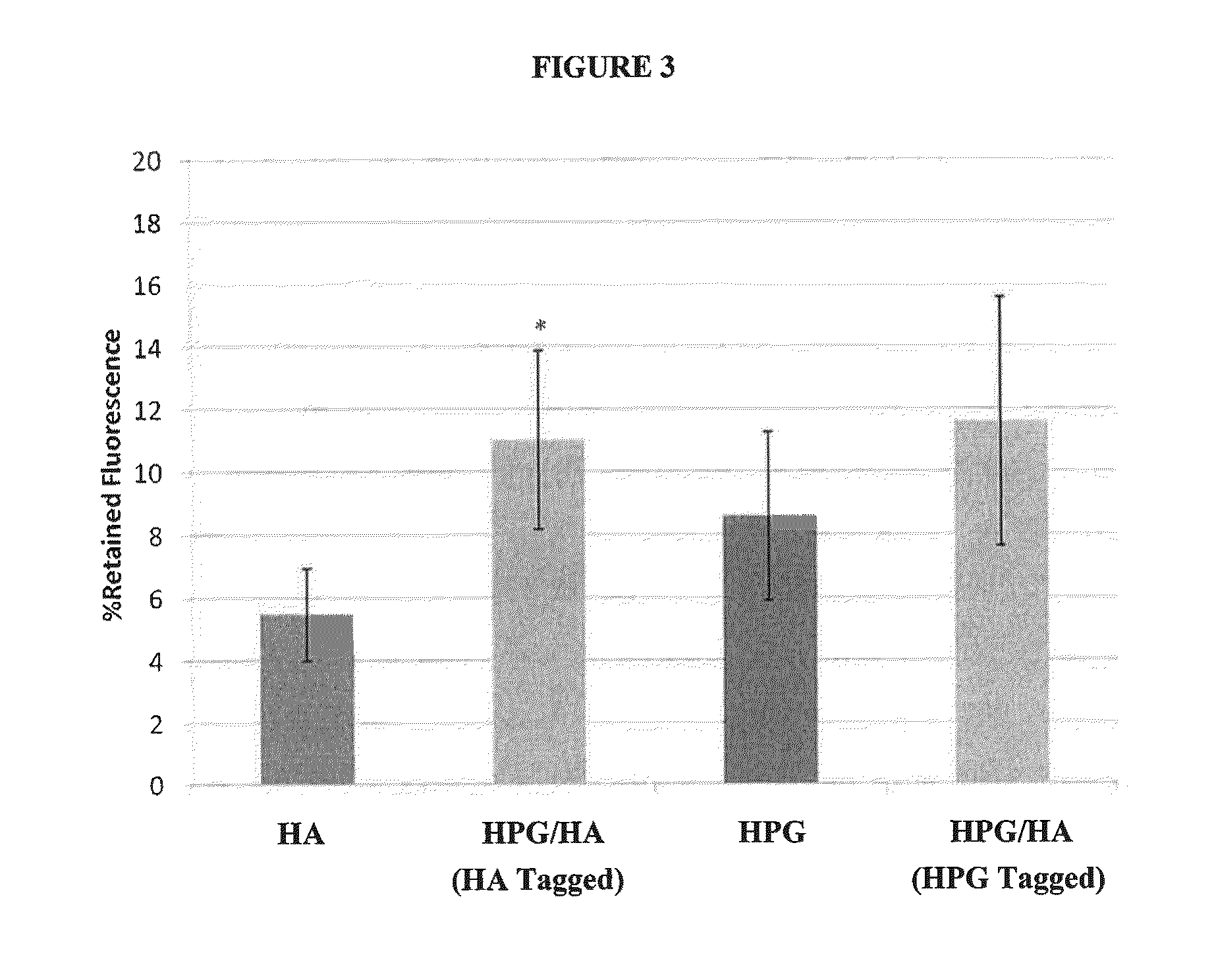
[0032]The ability of compositions of the present invention to protect human epithelial cells from desiccating stress was evaluated as follows. Human transformed corneal epithelial cells were plated at 0.09×106 cells / mL onto collagen-coated 48-well plates (BD Biosciences #35-4505) and grown to confluence in EpiLife media (Invitrogen #MEPI500CA) supplemented with Human Corneal Growth Supplement (HCGS Invitrogen #S0095) for 48 hours. Cells were treated with test solutions for 30 minutes at 37° C. then rinsed 1× (250 μL) with supplement free media. All solutions were gently removed and the cells were subjected to desiccation at 45% humidity, 37° C. for 30 minutes in a desiccation chamber (Caron Environmental Chamber 6010 Series). Cellular viability was determined using an MTS assay (Promega # G5421) to calculate % protection relative to media control. An assessment of solution cell surface retention was conducted by modifying the above desiccation experiment whereby five “media washes” ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com