Absorption of therapeutic agents across mucosal membranes or the skin
a technology of mucosal membranes and therapeutic agents, applied in the direction of aerosol delivery, immunological disorders, drug compositions, etc., can solve the problems of poor absorption of therapeutic agents of large molecular weight and/or high hydrophilicity, and poor absorption of therapeutic agents across mucosal membranes. , to achieve the effect of improving the absorption of therapeutic agents, avoiding degradation by stomach acid, and improving absorption ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
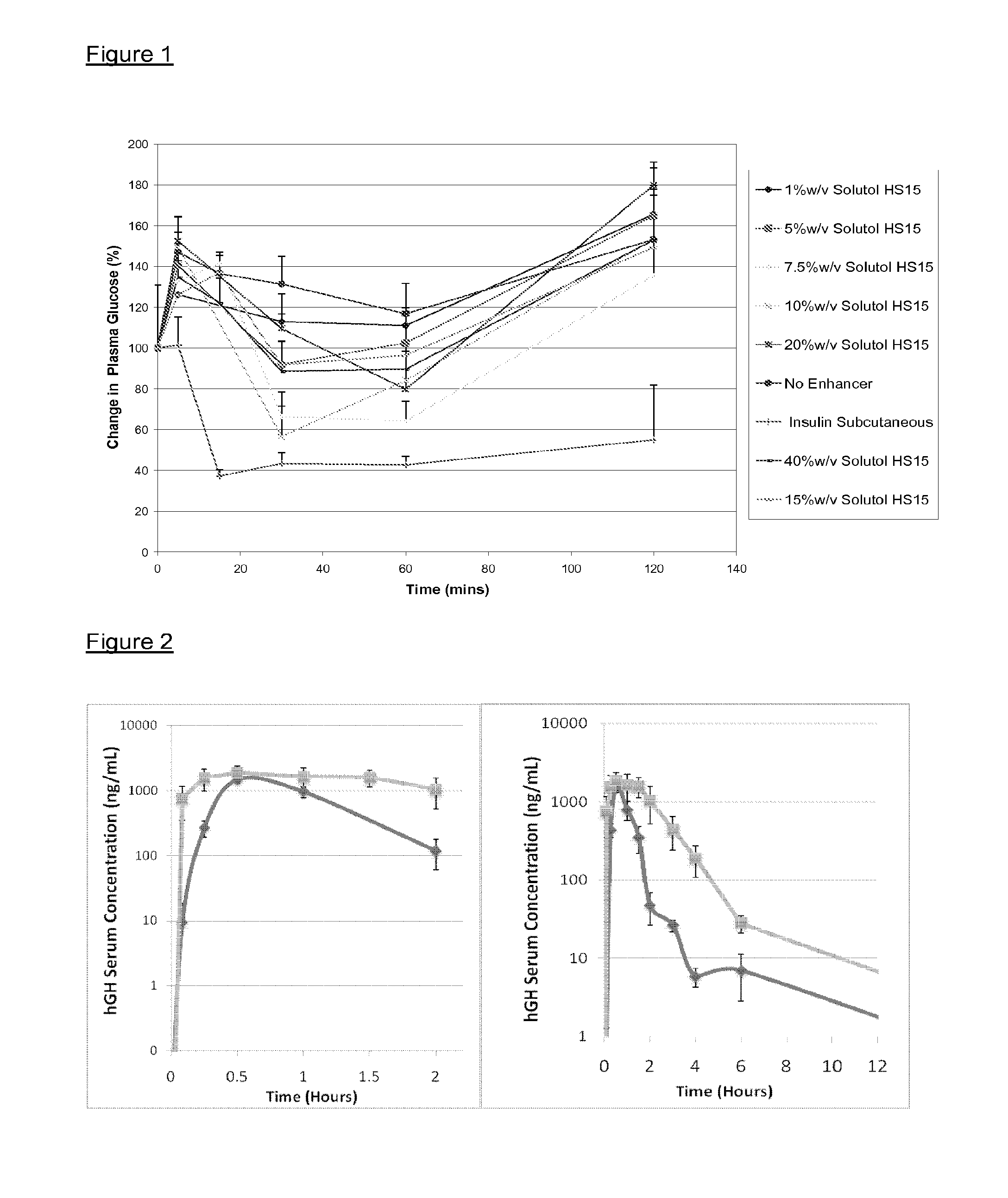
[0124]Insulin (available from Sigma-Aldrich) was dissolved in varying concentrations of Solutol® HS15 solutions ranging from 1 to 40% w / v in 0.063M phosphate buffered saline (PBS). The formulations were then administered intranasally to Sprague Dawley rats at 4 IU / kg and blood samples taken at frequent intervals up to 2 hours after administration. Blood glucose was measured using a standard glucose meter and insulin determined in the plasma by an enzyme linked immunosorbant assay (ELISA).
[0125]A group of rats was also administered with insulin dissolved in PBS via subcutaneous injection as comparison to the intranasal doses.
[0126]The results are shown in FIG. 1.
[0127]Solutol® HS15 formulations at 7.5% and 10% w / v in PBS were most effective at promoting the transport of insulin across the nasal mucosa showing a decrease in plasma glucose levels of 70%, as compared to control insulin solutions which showed a decrease of 15%.
example 2
[0128]Human growth hormone (hGH) (obtained from Bioker (Sardinia)) was dissolved in a 5% w / v Solutol® HS15 solution in PBS. The solution was then administered to Sprague Dawley rats intranasally at a dose rate of 5 mg / kg and compared to a subcutaneous administration of 5 mg / kg hGH. hGH levels in the serum were determined by enzyme linked immunosorbant assay (ELISA) up to 24 hours after administration.
[0129]The results are shown in FIG. 2.
[0130]The 5% w / v Solutol® HS15 formulation was effective at enhancing the transport of hGH across the nasal mucosa with a bioavailability of 17% compared to subcutaneous administration. The bioavailability of hGH administered nasally without an absorption enhancer is less than 1%.
example 3
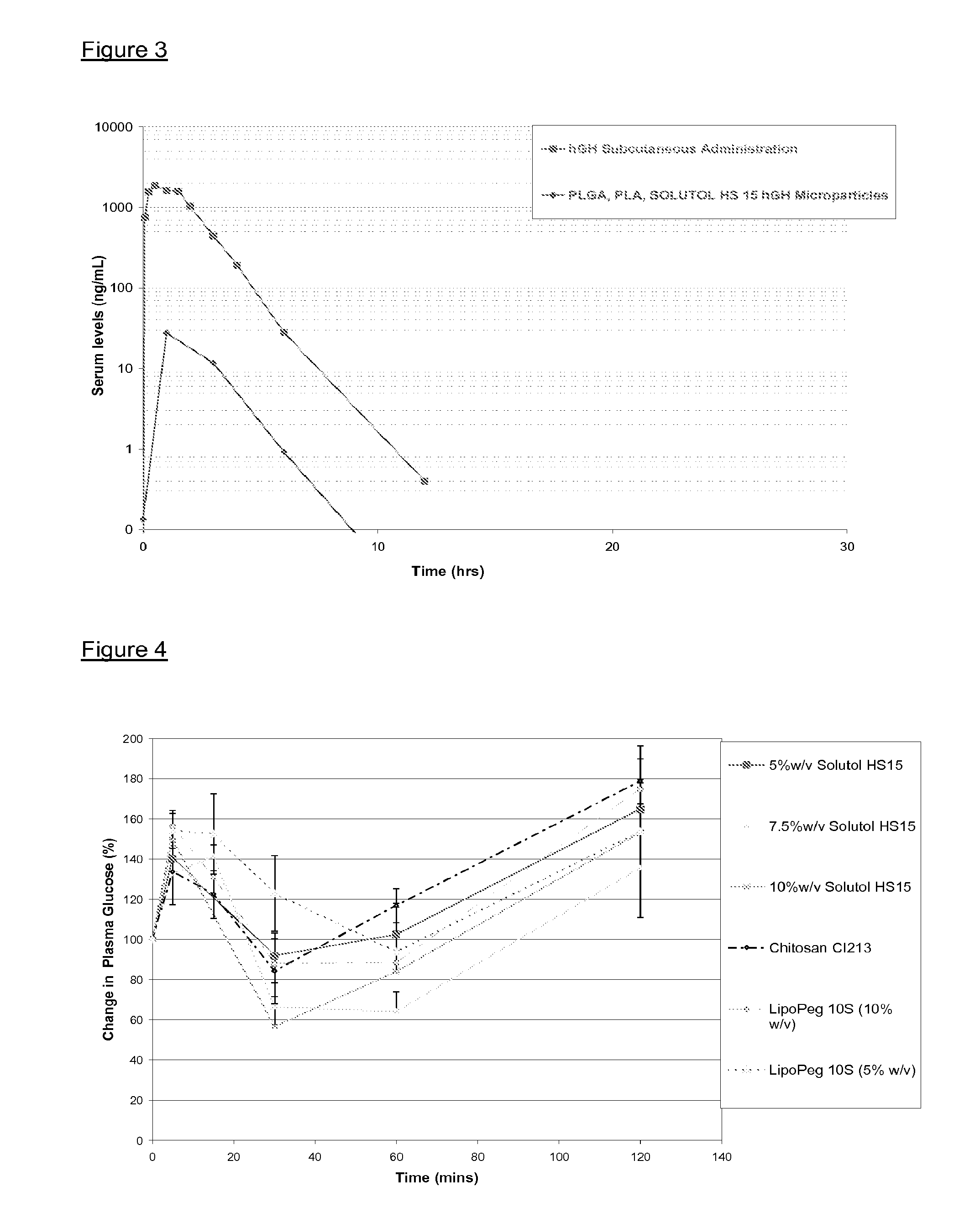
[0131]To prepare hGH loaded microparticles, micronised hGH, PLGA, PLA and Solutol® HS15 was added to a pressure vessel. The vessel was sealed and CO2 was introduced. The temperature was raised to above 32° C., and the pressure increased above 76 bar. Under these conditions CO2 becomes supercritical and dissolves into the polymer, which becomes liquefied. The liquefied polymer, hGH and Solutol® HS15 were then mixed and microparticles of a suitable size for injection were formed by solidification after the drug / polymer mixture was atomized and depressurised. hGH loaded microparticle formulations containing PLGA, PLA and PEG 600 were also prepared using the same method. The microparticles were administered to Sprague Dawley rats intranasally at a dose rate of 5 mg / kg hGH and compared to a subcutaneous administration of hGH. Blood samples were taken up to 24 hours after administration and hGH levels in the serum were determined by enzyme linked immunosorbant assay (ELISA).
[0132]The resu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| molecular weights | aaaaa | aaaaa |
| molecular weights | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com