System and method for image guided medical procedures
a medical and surgical technology, applied in the field of system and method for image guided medical and surgical procedures, can solve the problems of incontinence and erectile dysfunction, low tissue discrimination ability of ultrasound imaging, and serious side effects, so as to achieve the effect of minimizing corrections and effectively using them
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
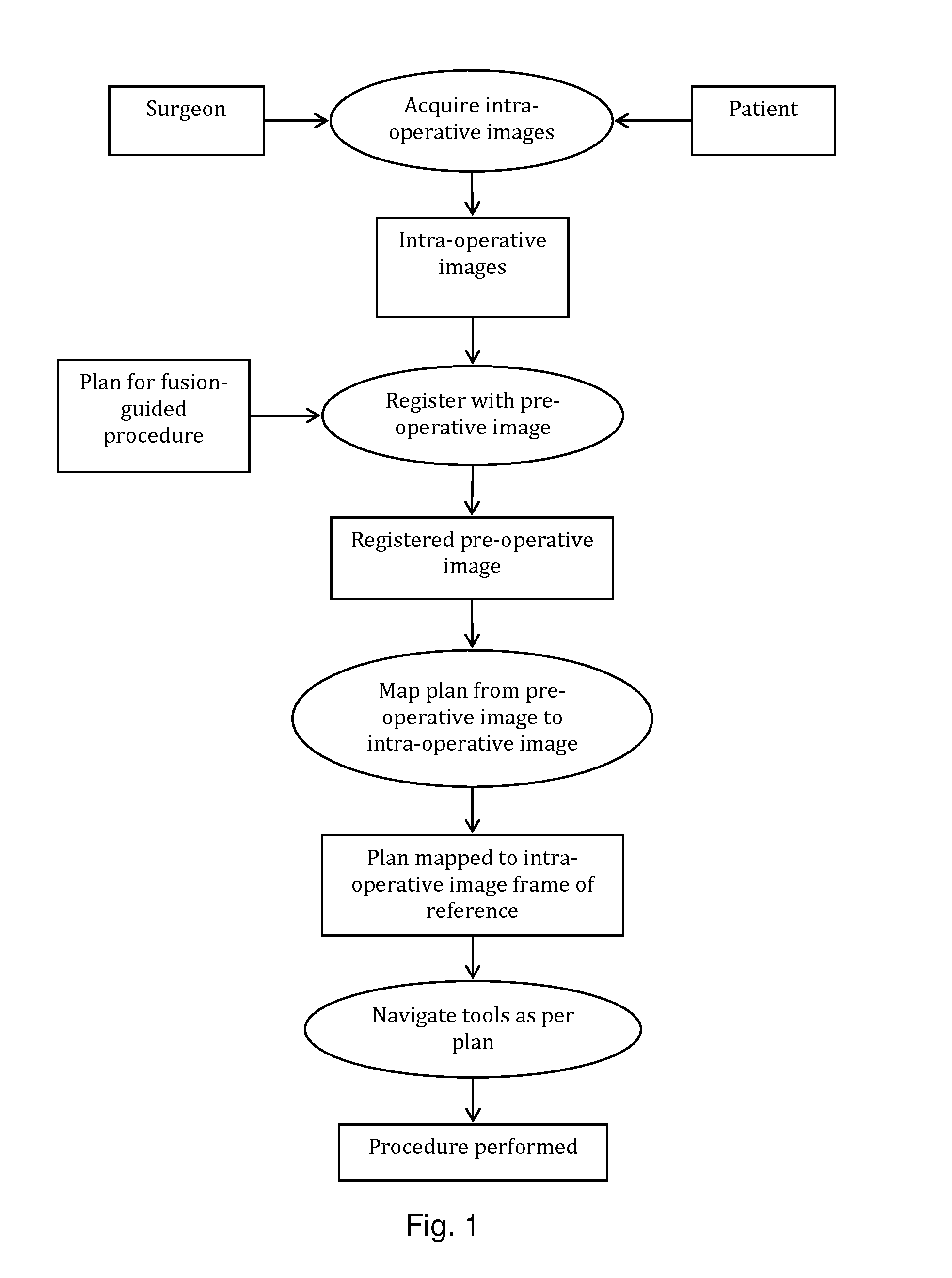
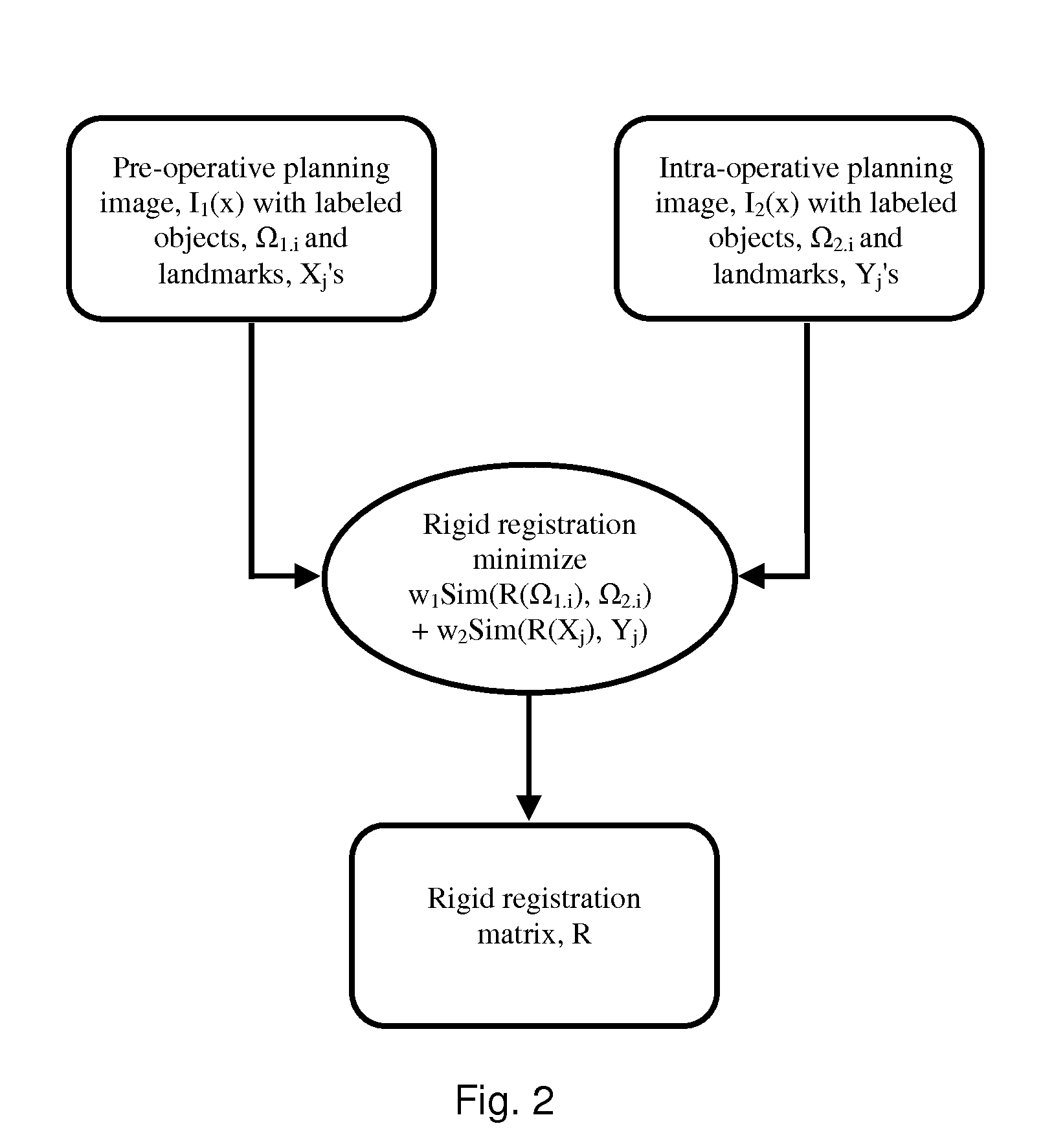
[0082]The present invention will be described with respect to a process, which may be carried out through interaction with a user or automatically. One skilled in the art will appreciate that various types of imaging systems, including but not limited to MRI, ultrasound, PET, CT, SPECT, X-ray, and the like may be used for either pre-operative or intra-operative imaging, but that a preferred scheme employs a fusion of MRI and / or CT and / or PET and ultrasound imaging for the pre-operative imaging, and trans-urethral ultrasound for intra-operative real time imaging in a prostate diagnosis or therapeutic procedure.
[0083]According to an embodiment of the present technology, one or more pre-procedure “planning” images are used to plan a procedure and one or more intra-procedure “live” images used to guide the procedure. For example, prostate biopsy and ablation is typically done under ultrasound guidance. While speed of imaging and cost make ultrasound an ideal imaging modality for guiding...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com