Topical Anti-inflammatory compositions and uses thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Formulation of Multi-Component Anti-Inflammatory Compositions
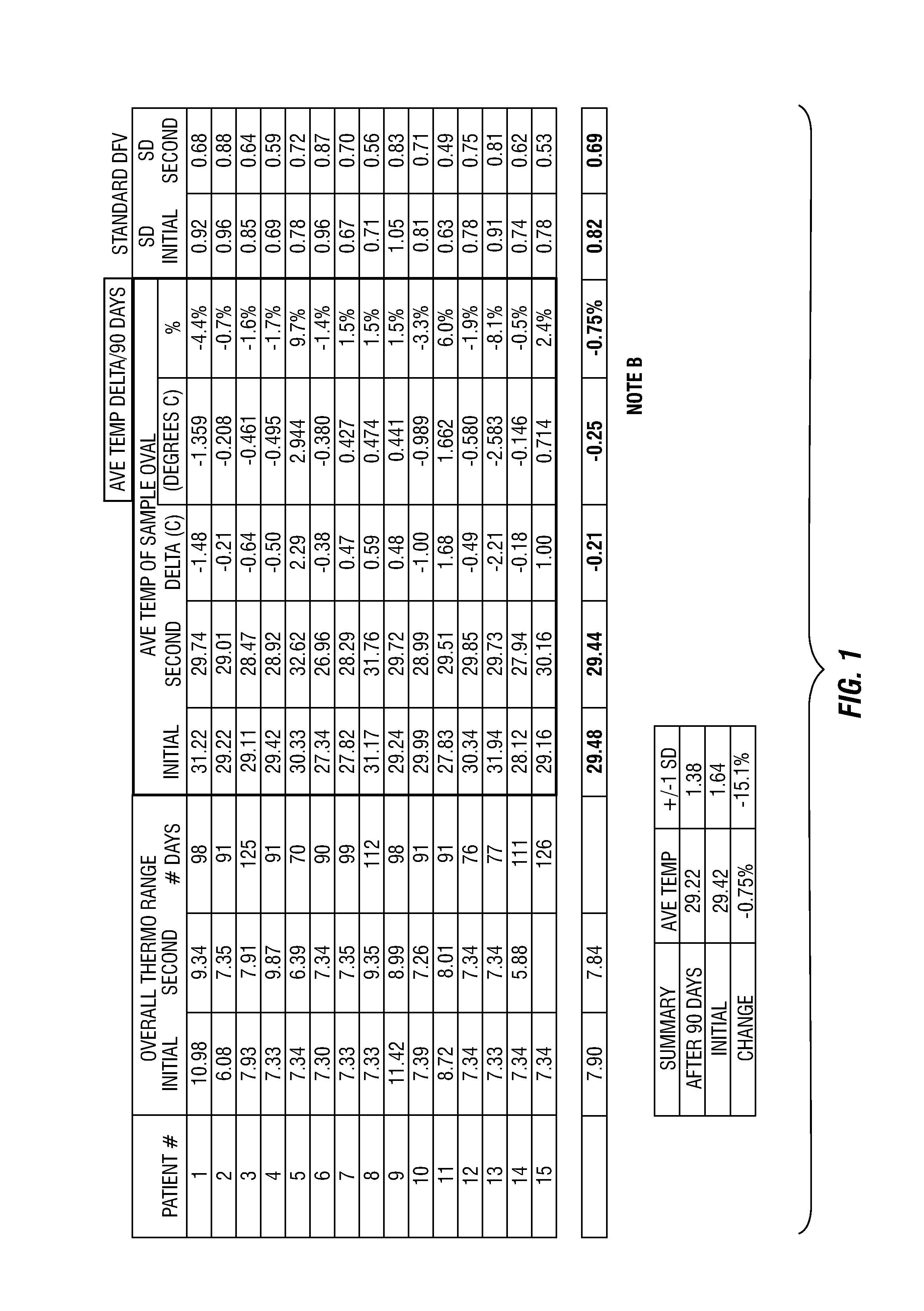
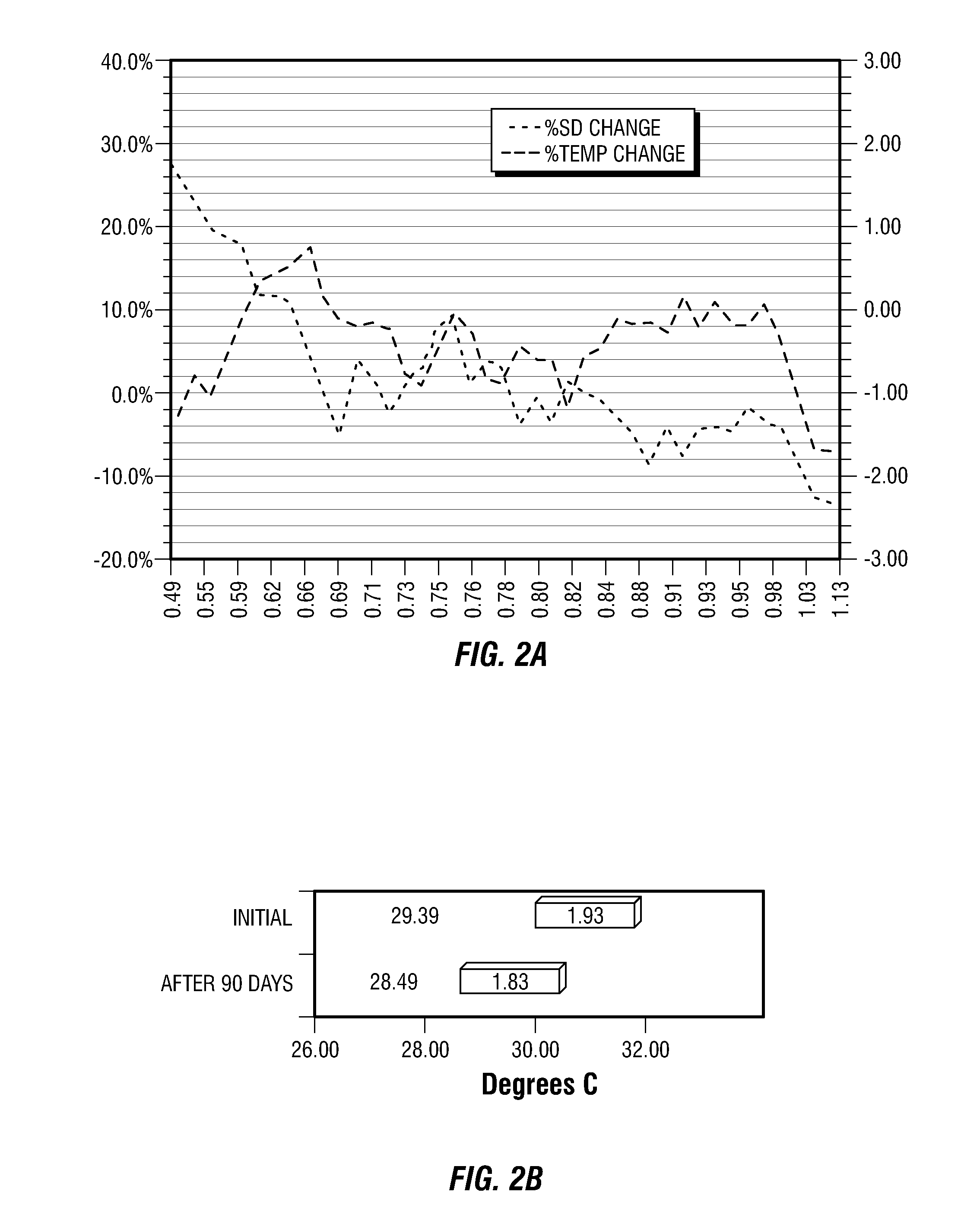
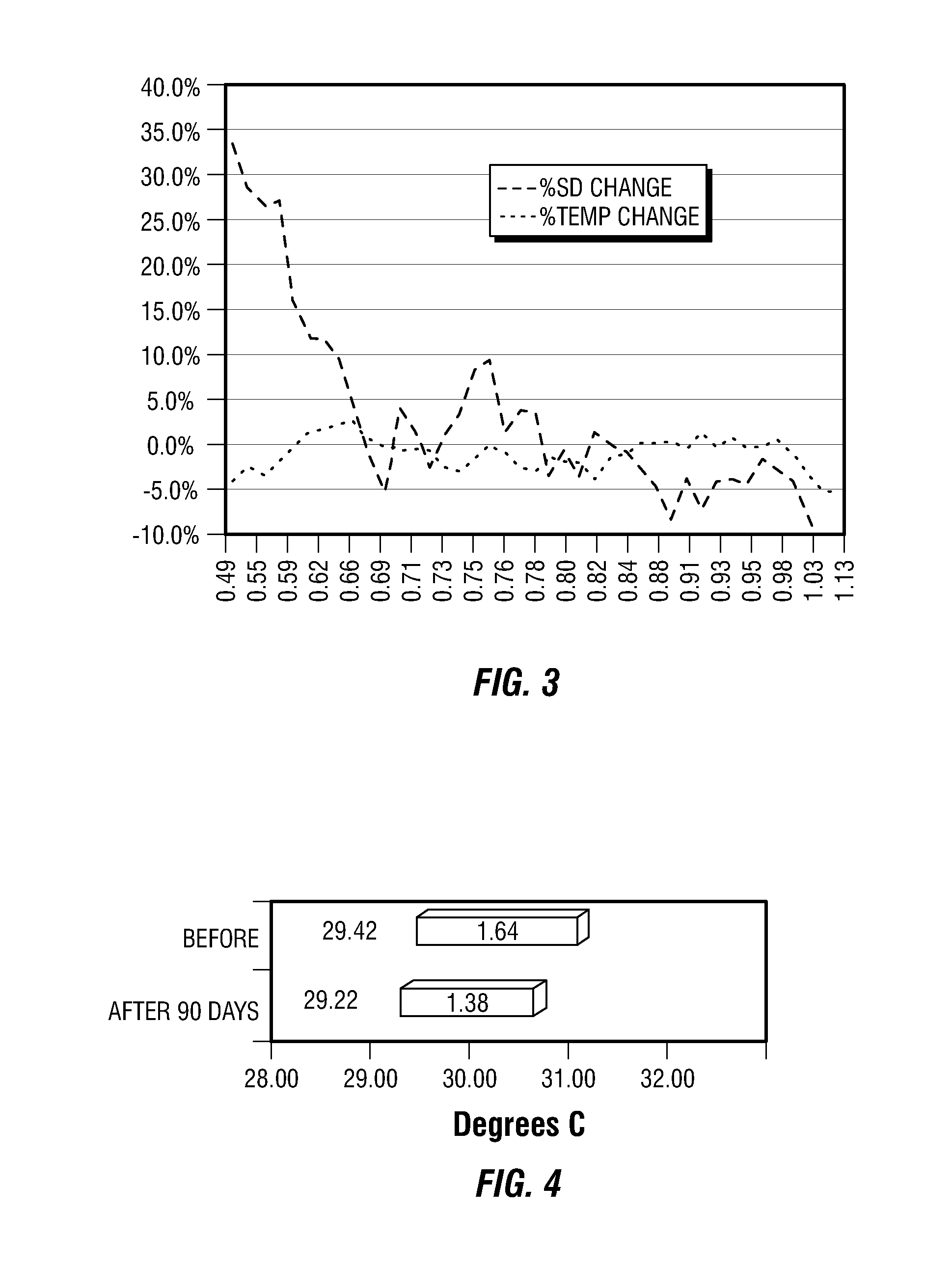
[0205]This example provides four, successively developed, formulations that include one or more of the various classes of orthomolecular reagents as described herein. Table 1 summarizes the components of each of these specific formulations.
TABLE 1IngredientFormula 1Formula 2Formula 3Formula 4WaterIsopropyl palmitateIsopropyl myristateLipowax D (cetearyl alcohol and ceteareth-20)Tetrahydrocurcumins (60 / 39 / 1020gtetrahydrocurcumin / tetrahydrocurcumins / tetrahydropiperine) (ascurcuminoid CG powder)α-Lipoic acid DL alpha (thioctic acid)5g0.15g5g5gKeltrol CG (xanthan gum)Lipoderm-core (PCCA Lipoderm)900g27g800g535gWasabia japonica (i-Sabi ™) powder20gBroccoli seed oil with conc. sulforaphane40mL1.2mL40mL40mL104 mg.MelatoninPharmasolve NPM (N-methyl pyrrolidone)Vitamin D326mL0.78mL26mL26mLVitamin E (tocopherol acetate)0.15mL5mL5mL3′3′-diindolylmethane (DIM) powder2gResveratrol (3,5,4′-trihydroxy-trans-stilbene)20g0.6g20g20gFrankinc...
example 2
Second- and Third-Generation Anti-Inflammatory Compositions
[0207]This example provides a component-by-component analysis of a second-generation formulation developed by the inventors that demonstrated particularly surprising and unexpected results in the practice of one embodiment of the present invention. Table 2 summarizes the active and inactive ingredients contained with specific formulation developed.
[0208]An exemplary, third-generation formulation has also been prepared that further includes (in addition to each of the components listed in Table 2), wintercress seed powder and wintercress seed oil (at final concentrations of 0.3% and 2%, respectively).
TABLE 2AN EXEMPLARY 2ND-GENERATION ORTHOMOLECULAR FORMULATION%Ingredient(wt. / wt.)FamilyPHASE AWater60PHASE BIsopropyl palmitate3Isopropyl myristate3Lipowax D (cetearyl alcohol and ceteareth-20)5SabiWhite ™ (tetrahydrocurcumin from Curcuma longa)0.25CurcuminoidsTetrahydrocurcuminoids CG ™ (standardized power from0.25CurcuminoidsCu...
example 3
Exemplary Procedure for Formulating Topical Creams
[0209]This example describes one exemplary procedure for formulating a topical cream (FIG. 6A and FIG. 6B) in accordance with the present invention:
[0210](1) Combine ingredients of PHASE A, and heat to ˜75-80° C. with mixing; (2) In a separate vessel, combine ingredients of PHASE B, and heat to ˜75-80° C. with mixing; (3) In a separate vessel, combine ingredients of PHASE C, and mix using a mixing propeller. Heat to ˜60° C. with mixing; (4) In a separate vessel, combine ingredients of PHASE D and mix well until solubilized; (5) When “PHASE A” and “PHASE B” each reach a temperature of ˜75-80° C., add PHASE A to PHASE B, and homogenize for 2 min at 2,000 rpm; (6) Using a mixing propeller, mix and maintain temperature for 10 min; (7) Begin cooling mixture, and continue mixing at moderate speed; (8) At ˜60° C., add PHASE C, and continue mixing; (9) At ˜35° C., add PHASE D, and continue mixing; (10) At ˜32° C., add PHASE E (cyclodextrin s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com