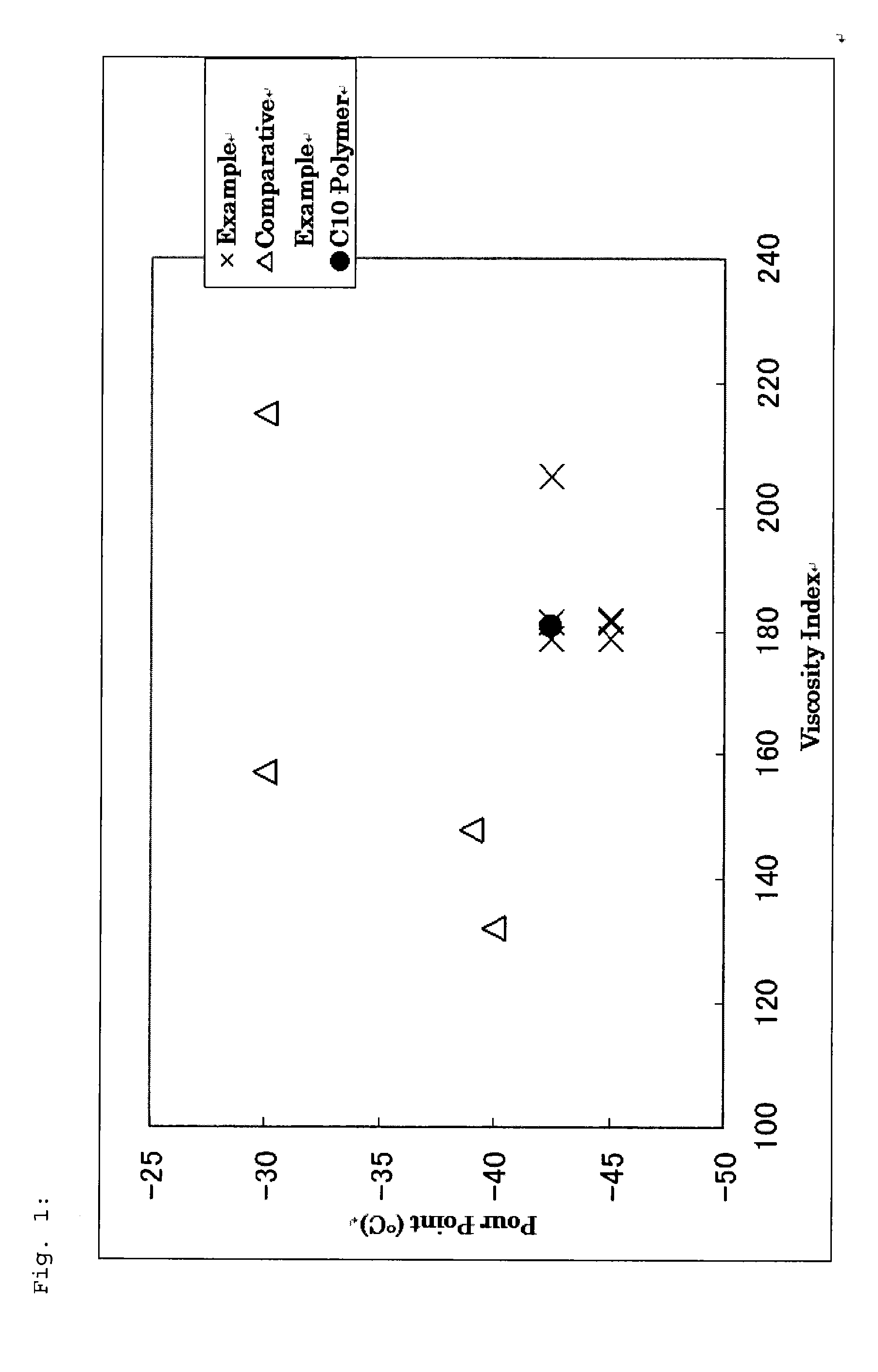
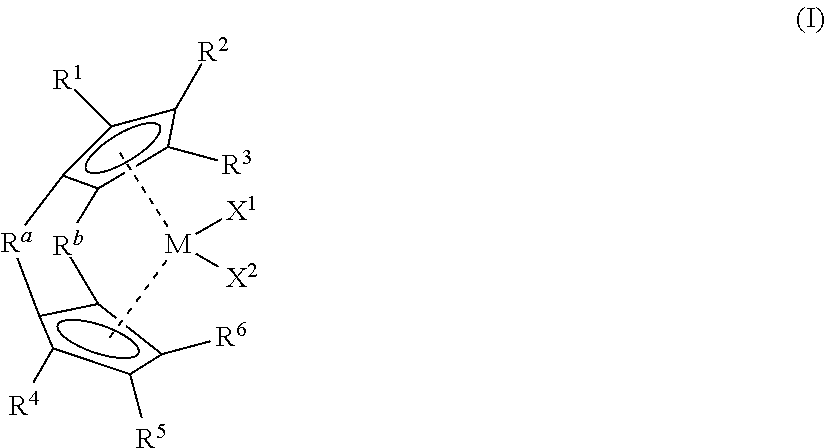1-octene, 1-decene, 1-dodecene ternary copolymer and lubricant composition containing same
a technology of octene and ternary copolymer, which is applied in the direction of lubricant composition, organic chemistry, fuels, etc., can solve the problems of high cost of -olefin polymer products for lubricant oil, high demand for 1-decene, and limited quantity of production of 1-decene, etc., to achieve excellent physical properties, low-temperature flowability and oxidation stability, excellent viscosity index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
production example 1
Production of (1,1′-dimethylsilylene)(2,2′-dimethylsilylene)-bis(cyclopentadienyl)zirconium dichloride
[0097]About 13.8 g (600 mmol) of metal Na and 400 ml of dry THF (tetrahydrofuran) were put into a nitrogen-purged 1000-ml three-neck flask, and stirred therein at 0° C. After 5 minutes, from 1 to 2 ml of cyclopentadiene was dropwise added thereto, and when the hydrogen generation was stopped, from 1 to 2 ml of cyclopentadiene was newly added thereto. This was repeated so that 50 ml (600 mmol) in total of cyclopentadiene was added. The reaction solution changed from colorless transparent to pale pink. After removal of THF through distillation under reduced pressure, the crystal was washed twice with hexane and dried into solid under reduced pressure to give cyclopentadienyl sodium as a pink powder.
[0098]457 ml of THF was added to 43.0 g (480 mmol) of cyclopentadienyl sodium and stirred at 0° C. This was cooled to −78° C., and 29.2 ml (480 mmol) of dichlorodimethylsilane was gradually...
example 1-1
[0104]A stainless autoclave having an inner capacity of 1 liter was fully dried and purged with nitrogen, and thereafter 146 ml of 1-dodecene, 183 ml of 1-decene, 71 ml of 1-octene, and next 0.2 mmol of triisobutylaluminium were put into it, and heated up to 105° C. One ml of a catalyst mixture prepared separately (this was prepared as follows: 0.1 mmol of triisobutylaluminium (2 mmol / ml toluene solution; 0.05 ml), 10 of (1,1′-dimethylsilylene)(2,2′-dimethylsilylene)-bis(cyclopentadienyl)zirconium dichloride prepared in Production Example 1 (10 mmol / ml toluene solution; 1 ml) and 0.012 mmol (11.5 mg) of powdery N,N-dimethylanilinium tetrakis(pentafluorophenyl)borate were put into a 10-ml glass-made Schlenk bottle in a nitrogen atmosphere, and stirred for 1 minute or so, and then 0.5 ml of 1-octene was added thereto and further stirred at room temperature for 1 hour) was put into the autoclave, and 0.05 MPaG hydrogen was introduced thereinto to start polymerization. After 60 minutes ...
example 1-2
[0105]In the same manner as in Example 1-1 except that the polymerization temperature was 90° C., a colorless transparent polymer from which the components having 24 or smaller carbon atoms had been removed, and its hydrogenated product were obtained in an amount of 235 g each. These were analyzed according to the above-mentioned methods, and the results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar ratio | aaaaa | aaaaa |
| kinematic viscosity | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com