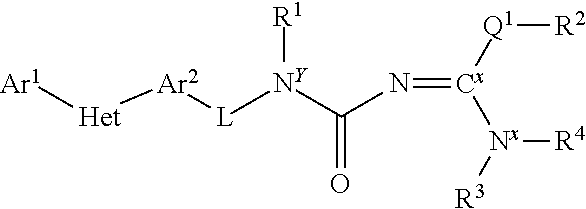
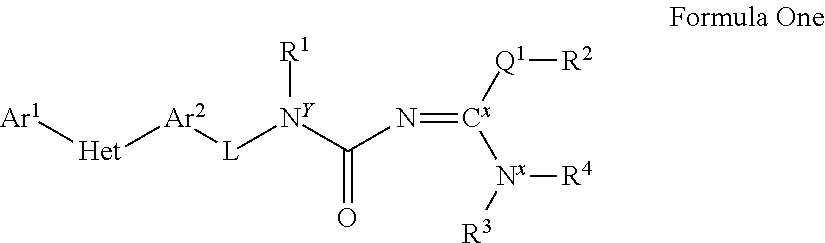
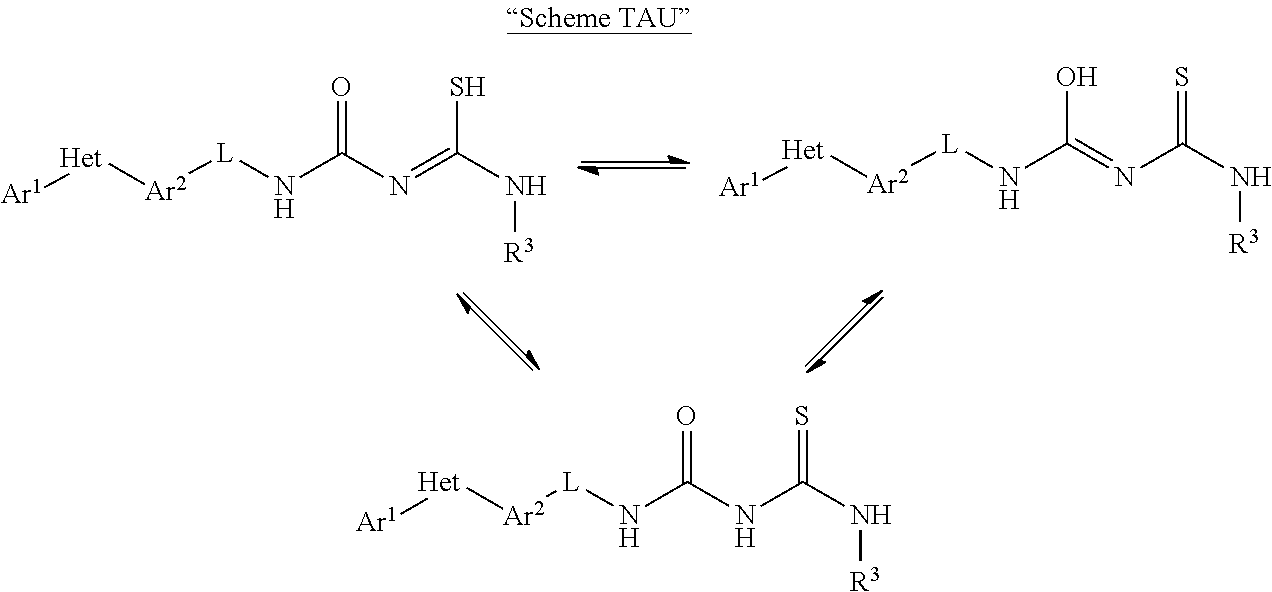
Molecules having certain pesticidal utilities, and intermediates, compositions, and processes related thereto
a technology of pesticidal utilities and molecules, applied in the field of molecules, can solve the problems of destroying more than 40% of all potential food production by weeds, affecting the quality of food, and often one of the most insidious and costly problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 3-bromo-1-(4-(trifluoromethoxy)phenyl)-1H-1,2,4-triazole (C1)
[0093]
[0094]To a 100 mL round bottomed flask, equipped with a stir bar, was added copper(I) iodide (0.397 g, 2.08 mmol), 3-bromo-1H-1,2,4-triazole (4.62 g, 31.2 mmol), and cesium carbonate (6.79 g, 20.83 mmol), as solids. These solids were diluted with anhydrous dimethyl sulfoxide (34.7 mL). Then 1-iodo-4-(trifluoromethoxy)benzene (1.65 mL, 10.4 mmol) was added as a liquid. The flask was placed under nitrogen atmosphere, and the suspension was heated to an internal temperature of 100° C. for 20 hours. The reaction mixture was allowed to cool to room temperature and filtered through a pad of Celite®, washing with excess ethyl acetate (200 mL). The filtrate was poured into a brine solution (200 mL), and the layers were partitioned. The aqueous phase was extracted with additional ethyl acetate (2×100 mL). The combined organic layers were dried over anhydrous magnesium sulfate, filtered, and concentrated. The re...
example 2
Preparation of methyl 2-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)acetate (C2)
[0100]
[0101]To a 200 mL round bottomed flask, equipped with a stir bar, was added [1,1′-bis(diphenylphosphino)ferrocene]dichloropalladium(II) (0.799 g, 1.09 mmol), 4,4,4′,4′,5,5,5′,5′-octamethyl-2,2′-bi(1,3,2-dioxaborolane) (6.65 g, 26.2 mmol), and potassium acetate (4.28 g, 43.7 mmol) as solids. These solids were diluted with dioxane (100 mL). The flask was sealed and pumped and purged with nitrogen atmosphere. Then methyl 2-(4-bromophenyl)acetate (5.00 g, 21.8 mmol) was added. The reaction mixture was then warmed to an internal temperature of 70° C. for 6 hours. The reaction mixture was allowed to cool to room temperature, and was poured into a brine solution and the layers were partitioned. The aqueous phase was extracted with ethyl acetate (3×125 mL). The combined organic layers were dried over anhydrous magnesium sulfate, filtered, and concentrated. The resulting residue was purified via ...
example 3
Preparation of methyl 2-(4-(1-(4-(trifluoromethoxy)phenyl)-1H-1,2,4-triazol-3-yl)phenyl)acetate (C3)
[0102]
[0103]To a 200 mL round bottomed flask equipped with a magnetic stir bar was added 3-bromo-1-(4-(trifluoromethoxy)phenyl)-1H-1,2,4-triazole (C1) (3.45 g, 11.2 mmol), and methyl 2-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)acetate (C2) (3.71 g, 13.4 mmol). These reagents were diluted with dioxane (45.0 mL) and water (11.3 mL) and the resulting solution was sparged with nitrogen gas for 10 minutes. Tri-tert-butylphosphonium tetrafluoroborate (0.325 g, 1.12 mmol), palladium(II) acetate (0.126 g, 0.560 mmol) and cesium fluoride (3.40 g, 22.4 mmol) were added as solids. The flask was sealed and placed under nitrogen atmosphere. The reaction mixture was heated to an internal temperature of 60° C. The reaction mixture was allowed to cool to room temperature, and was poured into a brine solution and the layers were partitioned. The aqueous phase was extracted with ethyl acet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| stereoisomers | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com