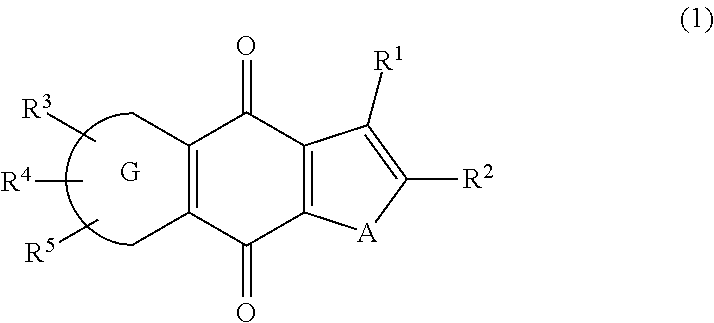
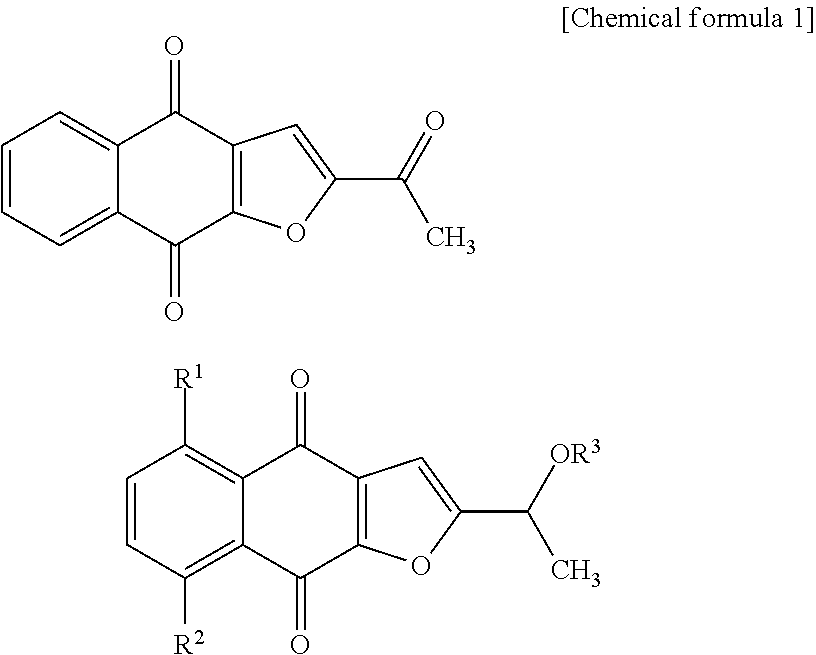
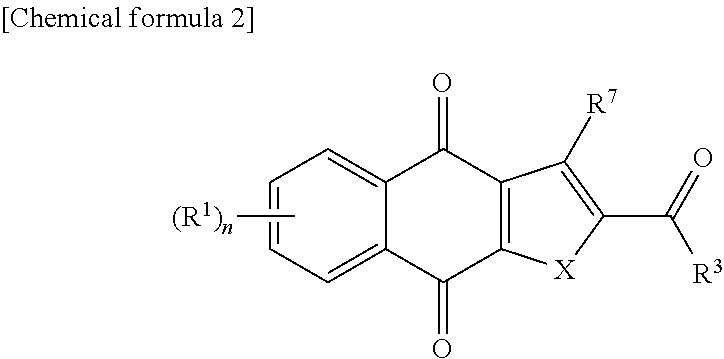
New tricyclic quinone derivative
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
methyl 4,7-dimethoxy-1-benzothiophene-2-carboxylate
[0204]
[0205]To a solution of 3,6-dimethoxy-2-nitrobenzaldehyde (70.0 g) in DMF (420 mL) was added dropwise methyl mercaptoacetate (33.4 mL) at room temperature. Subsequently, potassium carbonate (137 g) was added thereto in 10 parts, and then at unchanged constant temperature the reaction mixture was stirred for 1 hour. Methyl iodide (41.3 mL) was added dropwise thereto, followed by stirring for another 1 hour. The insoluble substance was removed through Celite, and then solvent was evaporated off. The resulting residue was dissolved in ethyl acetate (700 mL), washed with water (2×700 mL) and saturated brine (700 mL), and then dried over sodium sulfate. The solvent was then evaporated off to yield a standard compound as a flesh-colored powder (81.1 g).
[0206]1H-NMR (CDCl3, δ ppm): 8.04 (1H, s), 7.04 (1H, d, J=8.4 Hz), 6.90 (1H, d, J=8.4 Hz), 3.92 (3H, s), 3.89 (3H, s), 3.88 (3H, s) MS (ESI+) 253 (M++1).
reference example 2
methyl 5-amino-4,7-dimethoxy-1-benzothiophene-2-carboxylate (2A)
reference example 3
methyl 6-amino-4,7-dimethoxy-1-benzothiophene-2-carboxylate (2B)
[0207]
[0208]To a solution of methyl 4,7-dimethoxy-1-benzothiophene-2-carboxylate (2.04 g) (which was obtained in Reference example 1) in acetic acid (102 mL) was added dropwise 70% aqueous nitric acid solution (512 μL), and then the reaction mixture was stirred at 70° C. for 1 hour. After it was returned to room temperature, the reaction solution was poured into iced water, and then extracted with chloroform two times. The resulting organic layer was washed with saturated brine, and then dried over sodium sulfate. The solvent was then evaporated off to yield a mixture of 1A and 1B (2.57 g). This was used in the next reaction without further purification.
[0209]To a suspension of 90% reduced iron (2.68 g) and ammonium chloride (555 mg) in methanol / water (57.6 mL / 28.8 mL), which had been heated and stirred at 75° C., was added dropwise a suspension of the obtained mixture of 1A and 1B (2.57 g) in methanol (8.65 mL), and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com