Vaccine against acinetobacter baumannii based on cellular components deficient in lipopolysaccharide
a technology of lipopolysaccharide and vaccine composition, applied in the field of vaccine composition, can solve the problems of complicated clinical management of infections, complicated human use, and lack of clear evidence of bap expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Ethics Statement
[0141]All experiments involving the use of animals were approved by the University Hospital Virgen del Rock) Committee on Ethics and Experimentation (Evaluation code: 2013PI / 296). In all experiments, efforts were made to minimize suffering, and any animals appearing moribund during the course of experimentation were immediately euthanized using thiopental.
[0142]Bacterial Strains.
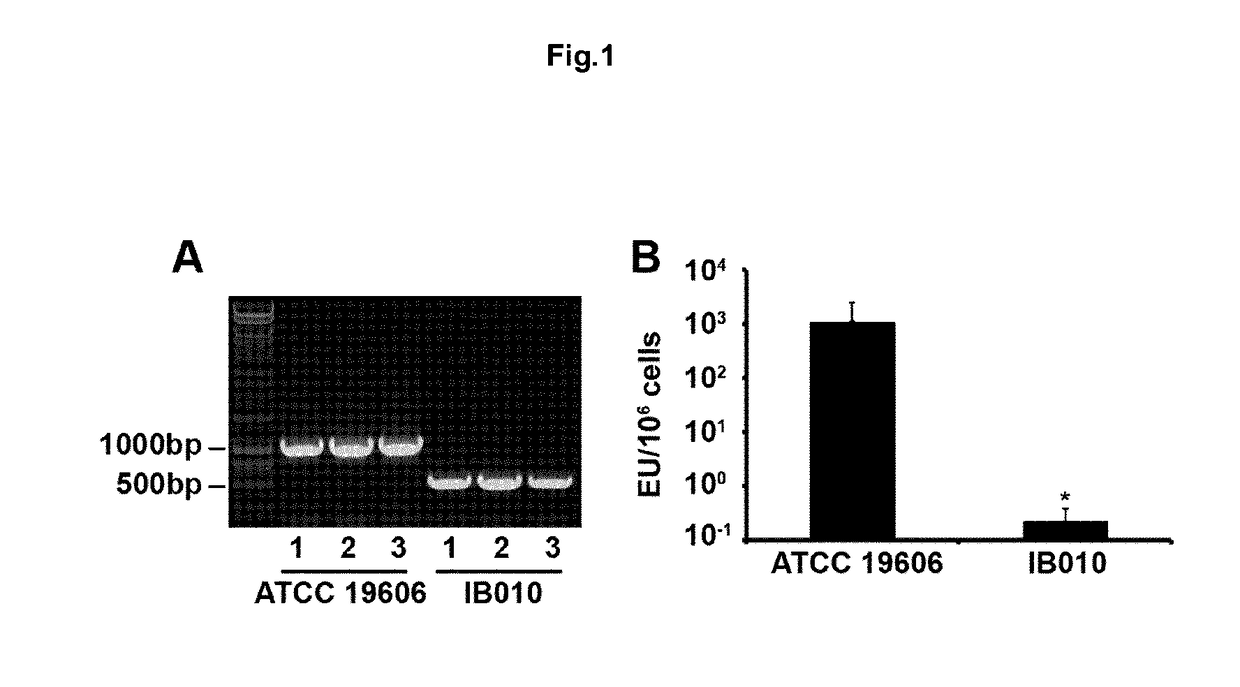
[0143]A. baumannii ATCC 19606 is an antibiotic susceptible reference strain. An LPS-deficient derivative of ATCC 19606 was obtained by plating an overnight culture of ATCC 19606 on Mueller Hinton agar containing 10 mg / I of colistin, as described previously (Clinical Laboratory Standards Institute 2013) Strains with mutations in the genes involved in LPS biosynthesis were identified by sequencing the IpxA, IpxC and IpxD genes of the colistin resistant mutants that were present after overnight growth at 37° C. A strain with a large deletion in the IpxD gene was identified and designated IB010. ...
example 2
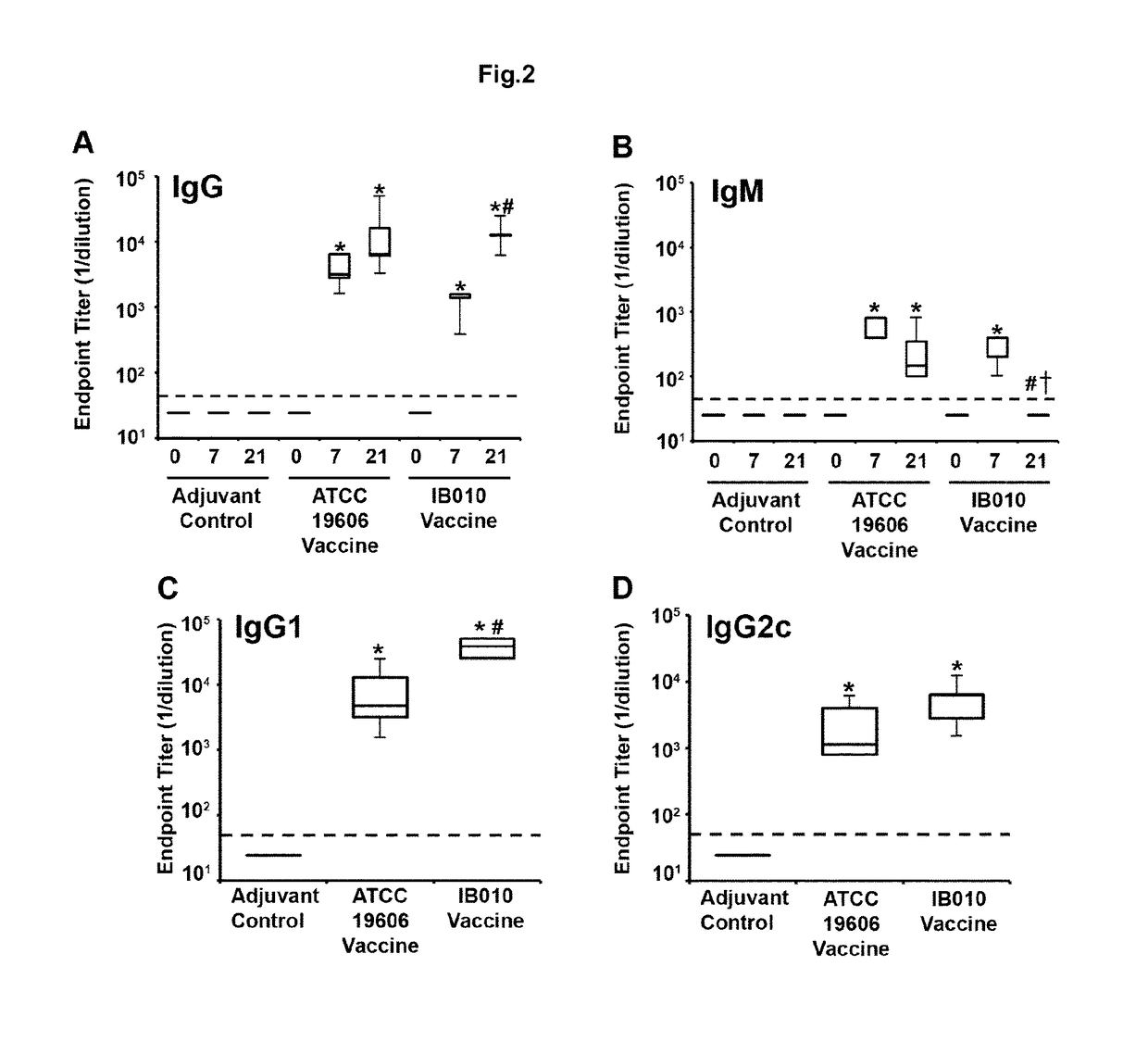
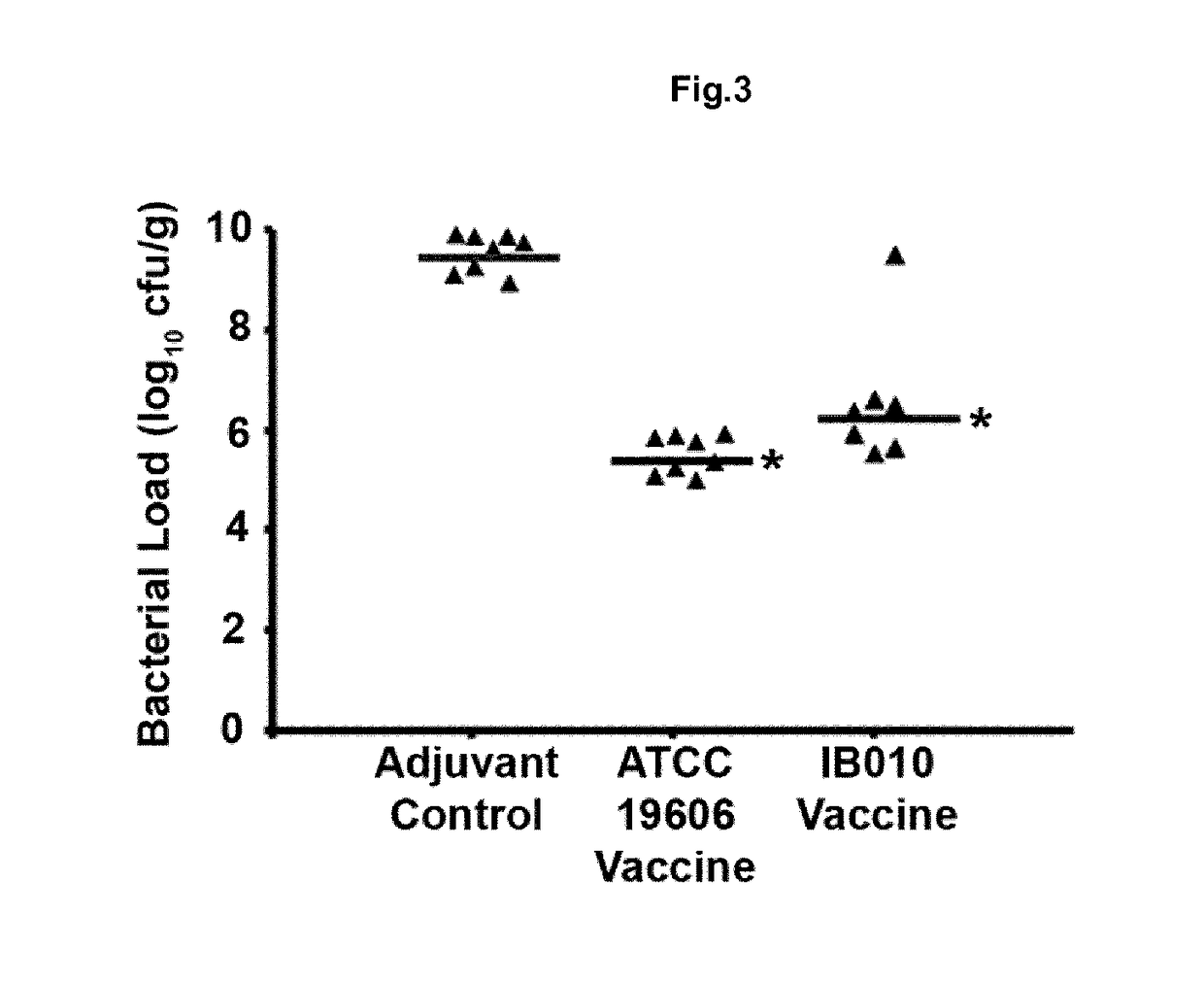
[0167]This example relates to the development of a vaccine against A. baumannii based on OMVS purified from said cultures of mutants without LPS.
[0168]To carry out this objective, the strains ATCC 19606T and its mutant without LPS IB010, which was generated in our laboratory from the ATCC 19606T strain and contains a deletion of 462 nucleotides between positions 103 and 565 of the gene IpxD.
[0169]Upon realizing the purification of the OMVs of said strains the following protocol was used:[0170]Strains are refreshed on blood agar or MHBII plates with colistin at 10 mcg / ml and grown overnight at 37° C.[0171]A liquid culture is used to growth ATCC 19606 or IB010. They are cultured with aeration at 180 rpm at 37° C. overnight.[0172]The next day cultures of 50 or 100 ml or 1 L in MHB are made and incubated overnight at 37° C. with aeration (180 rpm)[0173]After incubation, the cells are centrifuged at 4000 rpm during 30 min at 4° C.[0174]Next, the supernatant is filtered with a 0.22 micron...
example 3
Purification of Outer Membrane Proteins
[0182]A. baumannii ATCC 19606 was grown in 1 liter of Mueller-Hinton broth to an optical density at 600 nm (OD600) of 0.6, and pelleted bacteria were resuspended in 10 ml of 10 mM phosphate buffer, pH 7.2, and lysed by sonication. Unlysed cells were removed by centrifugation at 4,000×g for 5 min, and the supernatant was centrifuged at 20,000_g for 1 h to pellet cell envelopes. Inner membranes were selectively solubilized with 5 ml of 2% N-laurylsarcosinateby incubation at 37° C. for 30 min. The insoluble fraction was pelleted by centrifugation at 20,000_g for 1 h and then washed with 2 ml of 62.5 mM Tris-Cl, pH 6.8.
[0183]Endotoxin was extracted from the preparation by use of a cold detergent wash step in which proteins were resuspended in 5% SDS and incubated at 4° C. for 10 min. SDS and endotoxin were subsequently removed by precipitating in methanol chloroform and resuspended in PBS.
[0184]Addition of the Adjuvant
[0185]The purified proteins at...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| minimum inhibitory concentration | aaaaa | aaaaa |
| minimum inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com