Copper powder and electrically conductive paste, electrically conductive coating, electrically conductive sheet, and antistatic coating using same
a technology of copper powder and electrical conductive paste, applied in the field of copper powder, can solve the problems of increased seat weight, impaired flexibility of resin sheet, uneven dispersion state of metal filler in the resin, etc., and achieve excellent uniform dispersibility, suppressed increase in viscosity, and high electric conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0099]A titanium electrode plate having an electrode area of 200 mm×200 mm and a copper electrode plate having an electrode area of 200 mm×200 mm were installed in an electrolytic cell having a capacity of 100 L as the cathode and the anode, respectively, an electrolytic solution was put in the electrolytic cell, and a direct current was applied to this, thereby precipitating a copper powder on the cathode plate.
[0100]At this time, a solution having a composition in which the concentration of copper ion was 10 g / L and the concentration of sulfuric acid was 100 g / L was used as the electrolytic solution. In addition, polyethylene glycol (PEG) having a molecular weight of 400 (manufactured by Wako Pure Chemical Industries, Ltd.) was added as an additive to this electrolytic solution so as to have a concentration of 500 mg / L in the electrolytic solution, and a hydrochloric acid solution (manufactured by Wako Pure Chemical Industries, Ltd.) was further added thereto so that the concentra...
example 2
[0105]A copper powder was precipitated on the cathode plate under the same conditions as in Example 1 except that polyethylene glycol (PEG) having a molecular weight of 400 was added as an additive to the electrolytic solution so as to have a concentration of 1000 mg / L and a hydrochloric acid solution was further added thereto so that the concentration of chlorine ion was 50 mg / L.
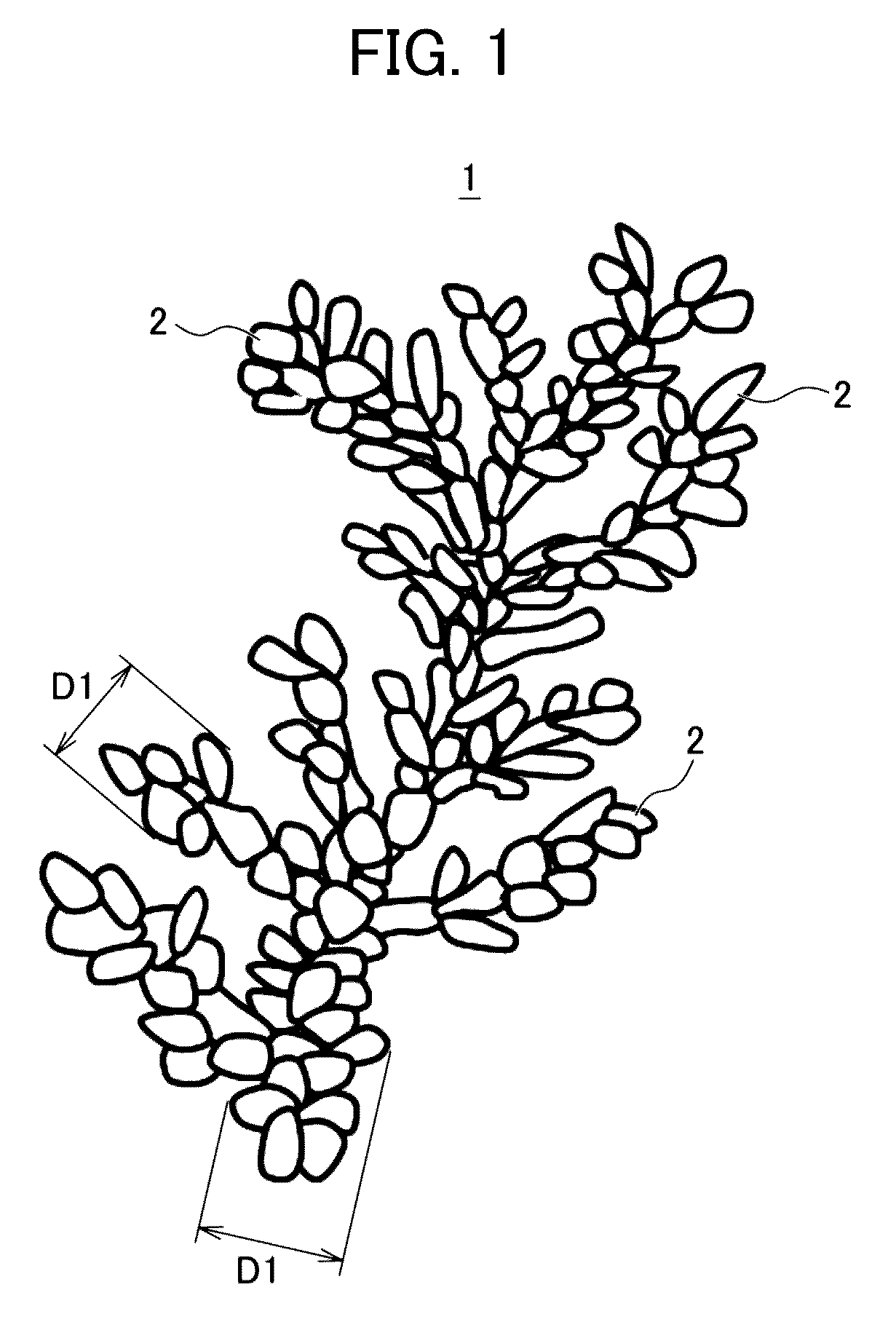
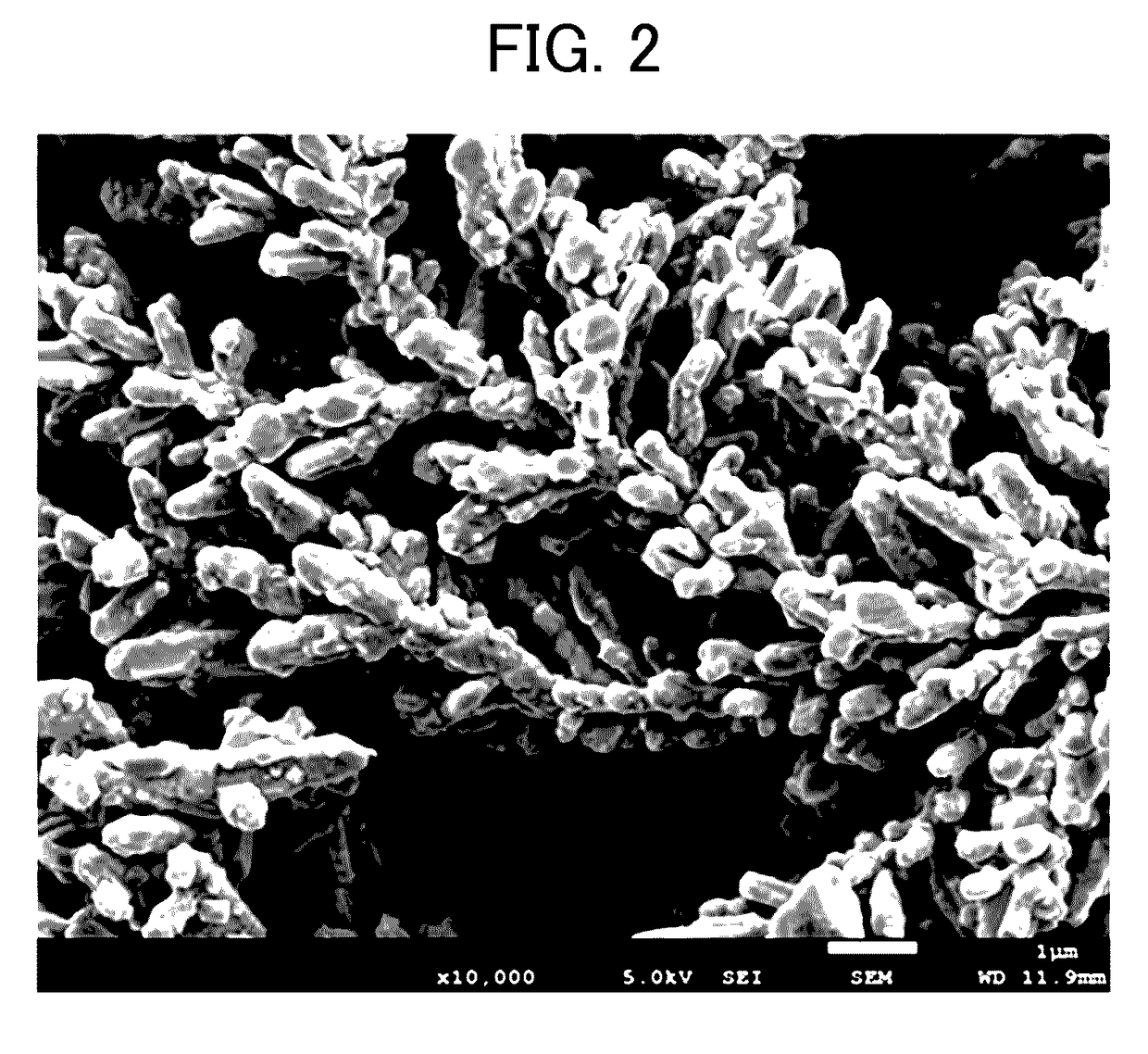
[0106]The shape of the electrolytic copper powder thus obtained was observed by the method using a scanning electron microscope (SEM) described above, and as a result, the copper powder thus precipitated was a copper powder having a dendritic shape constituted as elliptical copper particles having a size of from 0.2 μm to 0.5 μm in diameter and 0.32 μm in average thereof and from 0.5 μm to 2.0 μm in length and 1.4 μm in average thereof gathered. In addition, the crystallite diameter of the elliptical copper particles was 1956 Å.
[0107]In addition, the average particle diameter of the dendritic copper powder ...
example 3
[0108]A copper powder was precipitated on the cathode plate under the same conditions as in Example 1 except that the electric current was applied so that the current density of the cathode was 10 A / dm2.
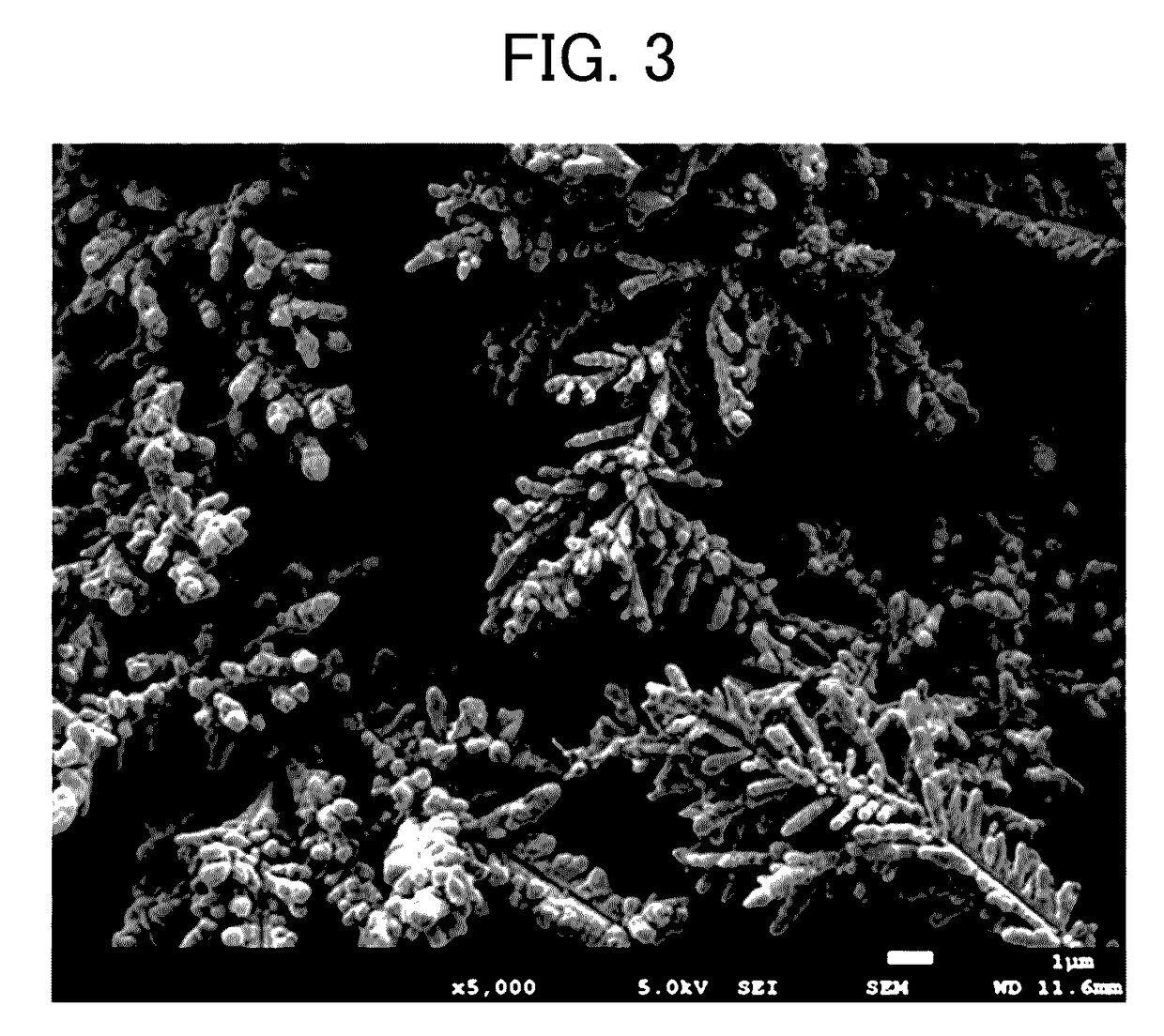
[0109]The shape of the electrolytic copper powder thus obtained was observed by the method using a scanning electron microscope (SEM) described above, and as a result, the copper powder thus precipitated was a copper powder having a dendritic shape constituted as elliptical copper particles having a size of from 0.2 μm to 0.5 μm in diameter and 0.48 μm in average thereof and from 0.5 μm to 2.0 μm in length and 1.8 μm in average thereof gathered. In addition, the crystallite diameter of the elliptical copper particles was 1105 Å.
[0110]In addition, the average particle diameter of the dendritic copper powder formed as the elliptical copper particles gathered was 18.2 μm. In addition, it was confirmed that a dendritic copper powder having a size of from 0.5 μm to 2.0 μm in thickness (di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com