Method for treating and preventing atherosclerosis and complications thereof
a technology for atherosclerosis and complications, applied in the field of atherosclerosis and complications thereof, can solve the problems of obesity, obesity syndrome, increased fat synthesis, etc., and achieve the effect of increasing the risk of vascular events
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Plasminogen Lowers Risk of Atherosclerosis Formation in 3% Cholesterol Hyperlipemia Model Mice
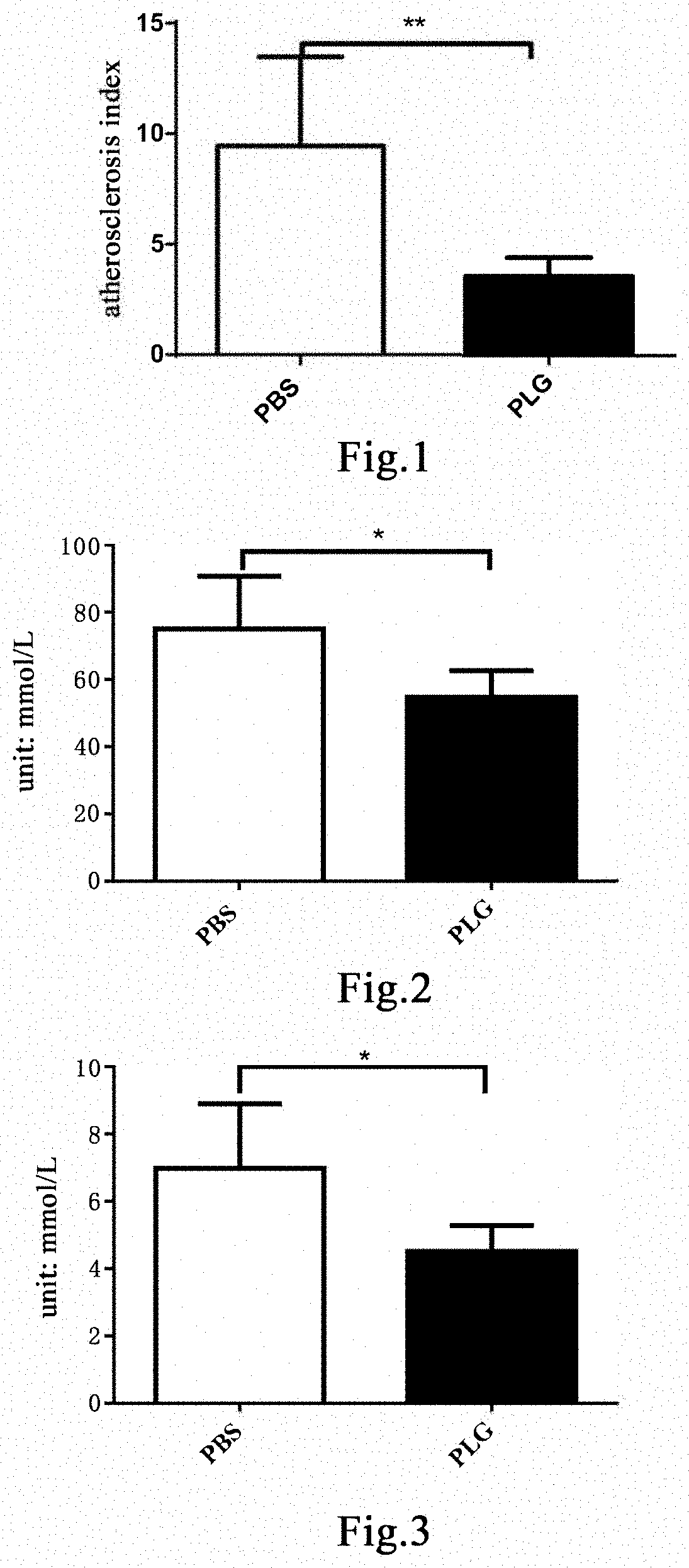
[0183]Sixteen 9-week-old male C57 mice were fed with a 3% cholesterol high-fat diet (Nantong TROPHIC) for 4 weeks to induce hyperlipemia[30,31]. This model was designated as the 3% cholesterol hyperlipemia model. The model mice continued to be fed with a 3% cholesterol high-fat diet. 50 μL of blood was taken from each mouse three days before administration, and the total cholesterol (T-CHO) was detected. The mice were randomly divided into two groups based on the total cholesterol concentration and the body weight, 8 mice in each group. The first day of administration was recorded as Day 1. Mice in the group administered with plasminogen were injected with human plasminogen at a dose of 1 mg / 0.1 mL / mouse / day via the tail vein, and an equal volume of PBS was administered to mice in the control group administered with vehicle PBS via the tail vein. After administration on Day 20, the mice beg...
example 2
Plasminogen Lowers the Content of Serum Total Cholesterol in ApoE Atherosclerosis Mice
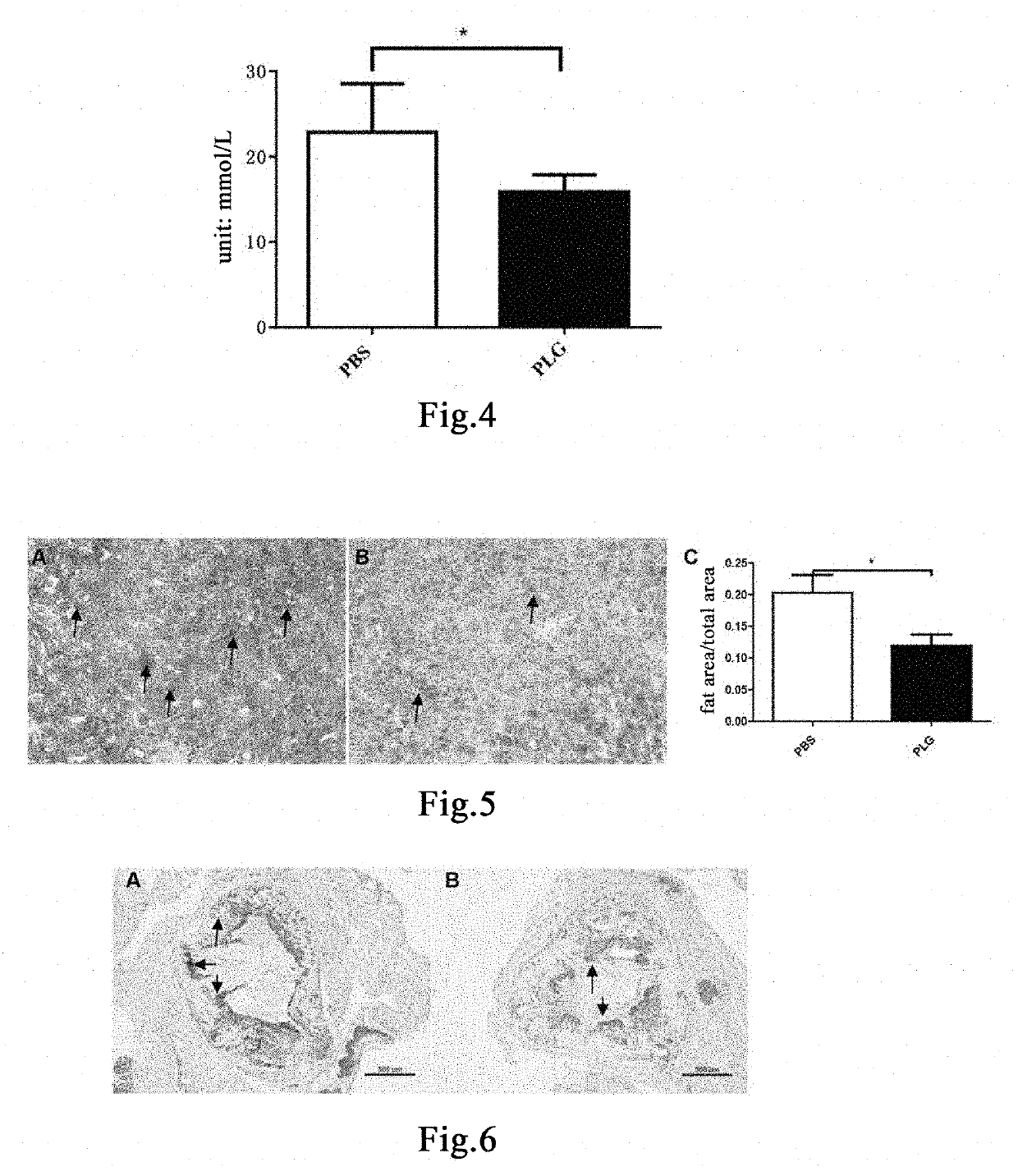
[0186]Thirteen 6-week-old male ApoE mice were fed with a high-fat and high-cholesterol diet (Nantong TROPHIC, TP2031) for 16 weeks to induce the hyperlipemia model[40, 41]. The model mice continued to be fed with a high-fat and high-cholesterol diet. 50 μL of blood was taken from each mouse three days before administration, and the total cholesterol (T-CHO) content was detected. The mice were randomly divided into two groups based on the T-CHO content, 7 mice in the control group administered with vehicle PBS, and 6 mice in the group administered with plasminogen. The first day of administration was set as Day 1. Mice in the group administered with plasminogen were injected with human plasminogen at a dose of 1 mg / 0.1 mL / mouse / day via the tail vein, and an equal volume of PBS was administered to mice in the control group administered with vehicle PBS via the tail vein, both lasting for 30 days. On ...
example 3
Plasminogen Lowers the Content of Serum Triglyceride in ApoE Atherosclerosis Mice
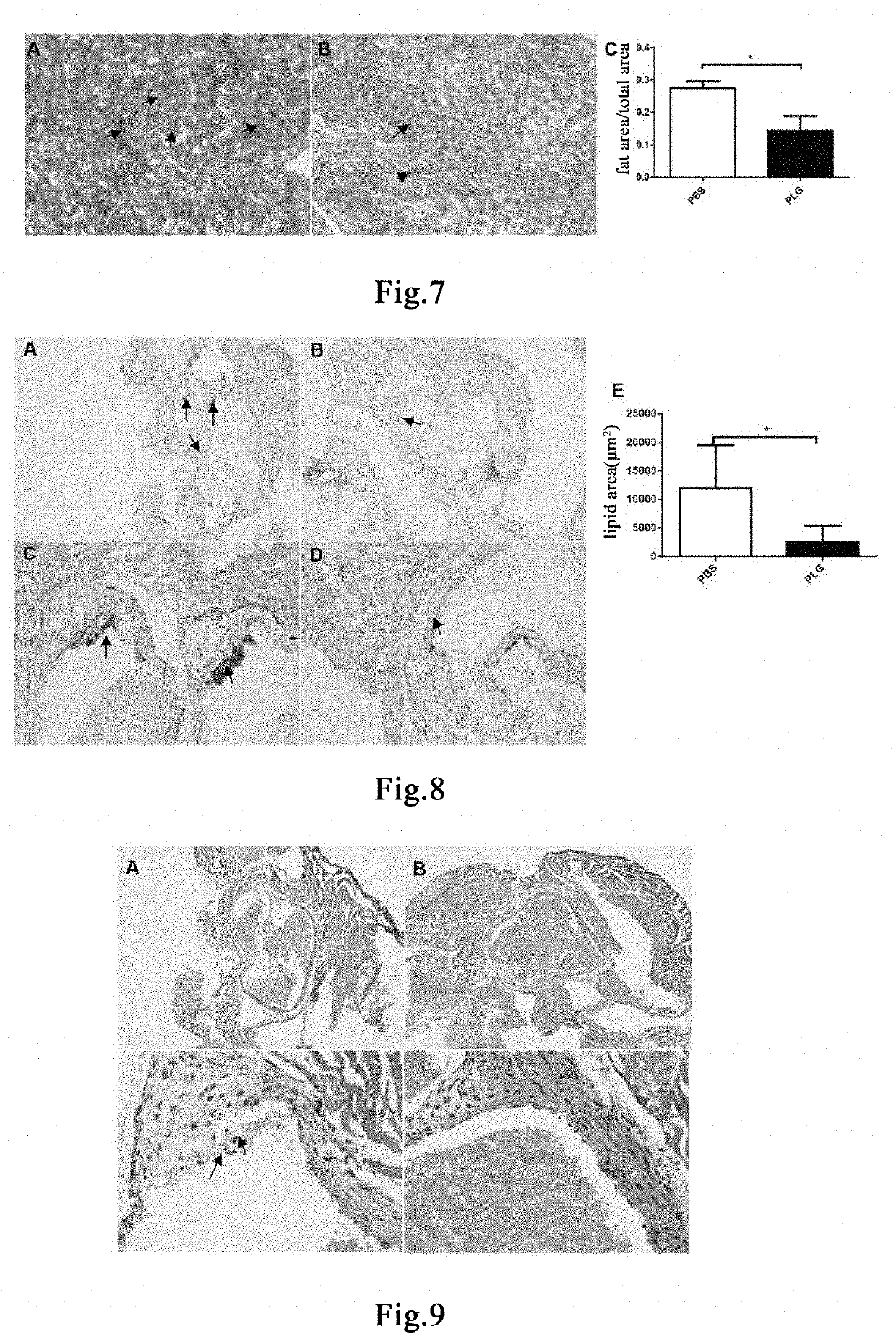
[0188]Thirteen 6-week-old male ApoE mice were fed with a high-fat and high-cholesterol diet (Nantong TROPHIC, TP2031) for 16 weeks to induce the hyperlipemia model[40, 41]. The model mice continued to be fed with a high-fat and high-cholesterol diet. 50 μL of blood was taken from each mouse three days before administration, and the total cholesterol (T-CHO) content was detected. The mice were randomly divided into two groups based on the T-CHO content, 7 mice in the control group administered with vehicle PBS, and 6 mice in the group administered with plasminogen. The first day of administration was recorded as Day 1. Mice in the group administered with plasminogen were injected with human plasminogen at a dose of 1 mg / 0.1 mL / mouse / day via the tail vein, and an equal volume of PBS was administered to mice in the control group administered with vehicle PBS via the tail vein, both lasting for 30 days. On ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
| energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com