Chemical Wall-Treatment Method That Reduces the Formation of Coke
a wall treatment and chemical technology, applied in the direction of solid-state diffusion coating, metallic material coating process, coating, etc., can solve the problems of coking phenomena, deterioration of the conductivity of the walls, and loss of head
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
emical Treatment of the Surface
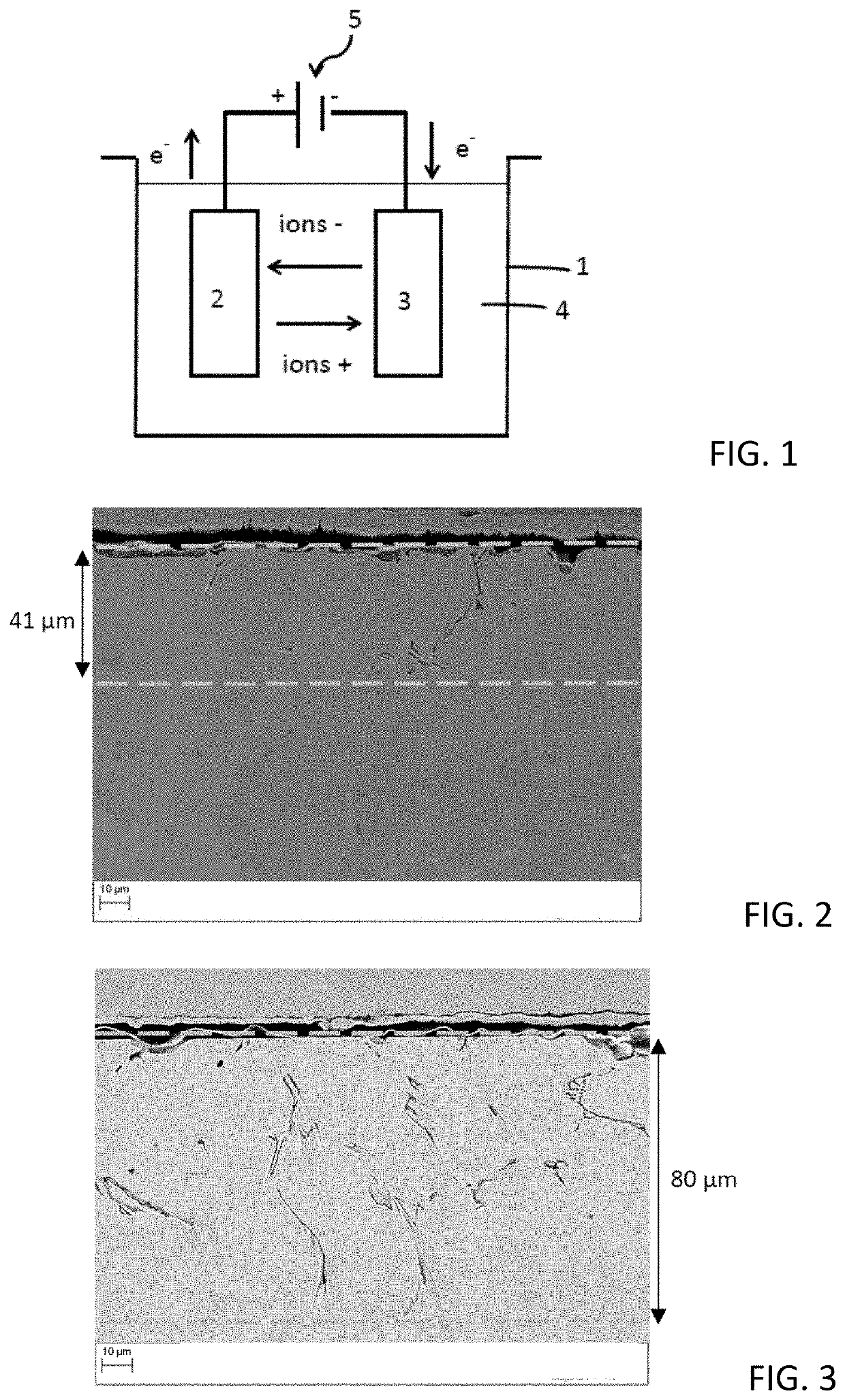
[0099]In this example, the sample is subjected to an electrolytic dissolution chemical treatment.
[0100]The sample to be tested is placed at the anode of an electrolysis cell, such as described in FIG. 1, the cathode being a metal plate made of stainless steel or of graphite, with dimensions similar to or greater than those of the sample. The anode and cathode are separated by a distance of approximately 1 cm, the plates being substantially parallel inside the electrolysis cell.
[0101]An electrolytic solution is prepared by dissolving, with mechanical stirring, 135 g of NaOH (in the form of pellets) in 1 l of distilled water and then the electrolysis cell is filled with the solution obtained. The chloride content of the solution is less than 10 ppm by weight.
[0102]A potential difference is applied between the anode (sample) and the cathode.
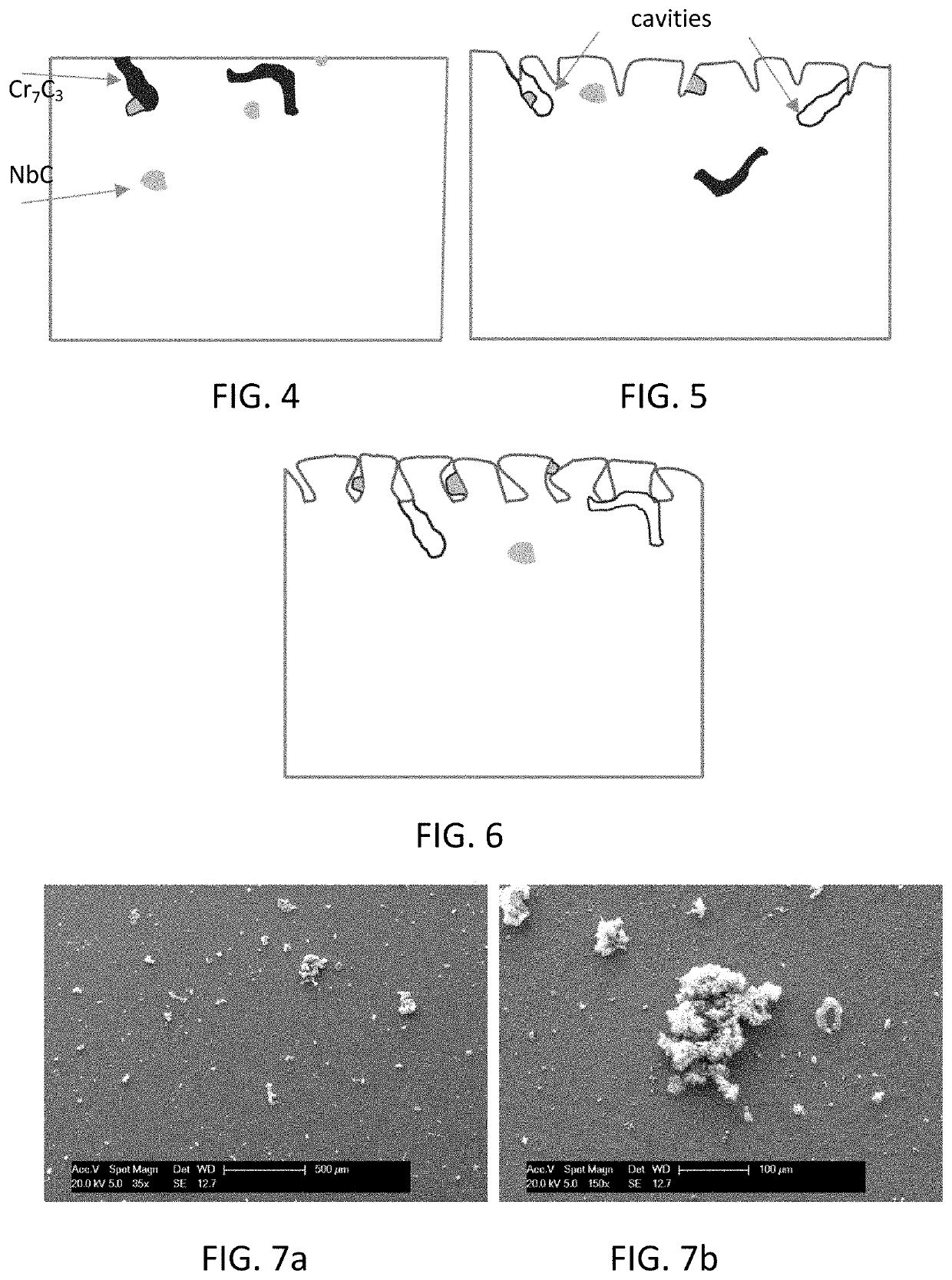
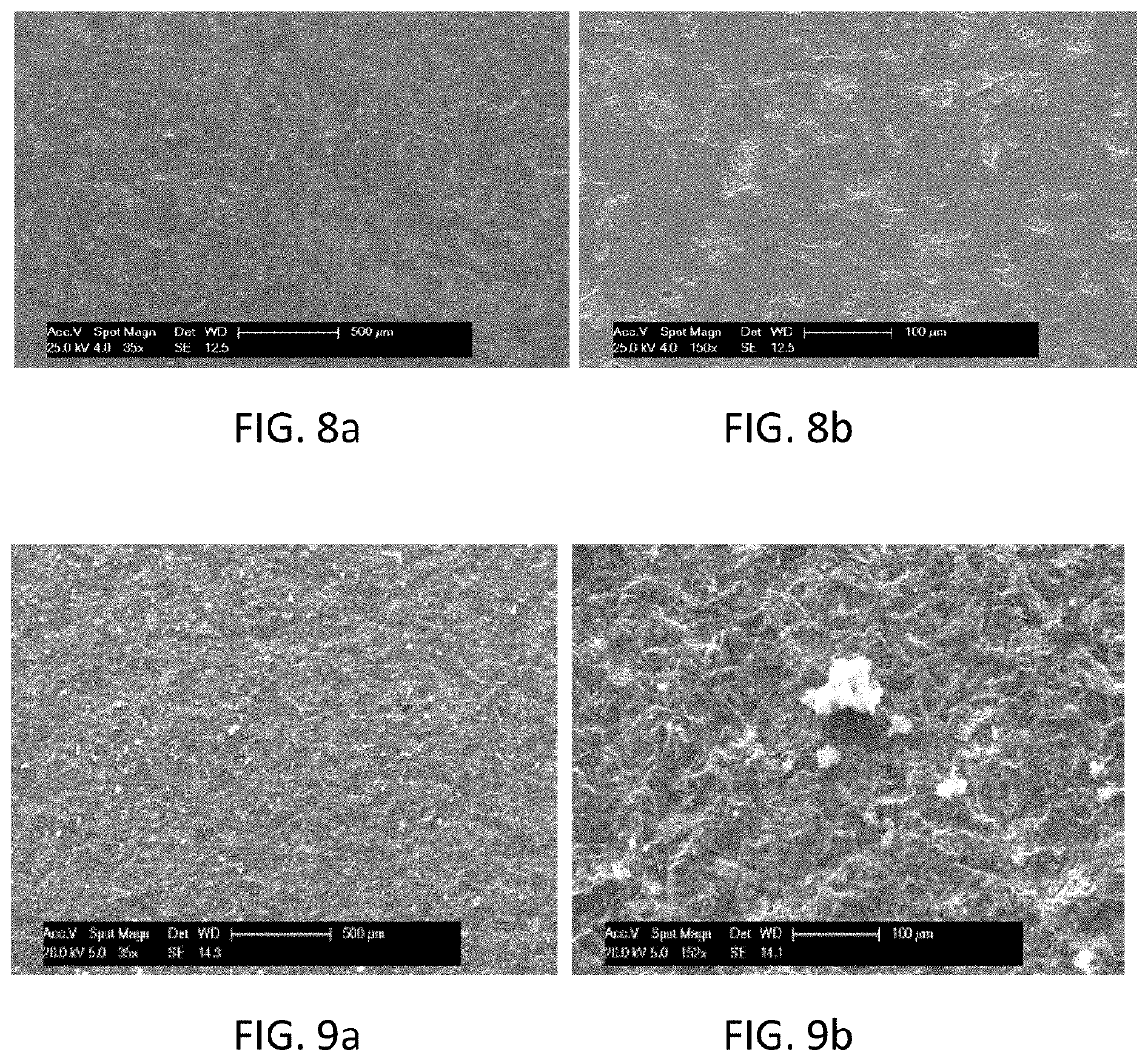
[0103]Two series of five and four tests were carried out on HP 25-35 alloys, which were all polished before being pl...
example 2
l Surface Treatment / Shot Peening
[0111]A polished HP 25-35 alloy sample is subjected to shot peening in a sleeve sandblasting chamber. The parameters used are as follows:[0112]Particles: glass beads Ø 100-200 μm[0113]Projection distance: approximately 15 cm[0114]Duration of the projection: 15 seconds for a sample of a few cm2 [0115]Carrier gas: compressed air under a controlled pressure of 2.5 to 3.5 bars, nozzle diameter 6 to 8 mm, 40 litres of particles in a closed circuit.
[0116]A sample M1 is obtained.
example 3
l Surface Treatment / Sandblasting (Alumina Blasting)
[0117]A polished HP 25-35 alloy sample is subjected to alumina blasting in a sleeve sandblasting chamber. The parameters used are as follows:[0118]Particles: brown corundum (Al2O3) Ø 250-400 μm[0119]Projection distance: approximately 15 cm[0120]Duration of the projection: 15 seconds for a sample of a few cm2 [0121]Carrier gas: compressed air under a controlled pressure of 2.5 to 3.5 bars, nozzle diameter 6 to 8 mm, 40 litres of abrasive particles in a closed circuit.
[0122]A sample M3 is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| depth | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com