Methods of screening for compounds that interact with human P2u2 purinergic receptor
a purinergic receptor and purinergic receptor technology, applied in the field of screening for compounds that interact with human p2u2 purinergic receptors, can solve the problems of difficult comparison of the relative potency of p.sub.2 -purinergic receptor agonists, the classification of sub.1 and p.sub.2 -type purinergic receptors is no longer sufficient to accurately portray this complex family of receptors, and the difficulty of sub.1 - and p.sub
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Isolation of Full-length Human cDNA encoding P.sub.2U2
Insert was isolated from the PCR clone of interest (206.18), purified from an agarose gel, radiolabeled with [.alpha.-.sup.32 P]dCTP(NEN) by random-priming (Stratagene), and used to screen a DAMI cDNA library in .lambda.gt22. The library was generated using twice-selected poly-A+ mRNA (see above) and first strand cDNA synthesis was primed with an oligo-dT primer and synthesized with Moloney murine leukemia virus (M-MLV) reverse transcriptase (Gibco / BRL). CDNA was directionally ligated into the SalI / NotI sites of the .lambda.gt22 arms and packaged (Stratagene packaging extract) and amplified in the Y1090 (r-) strain. One million clones were screened at a density of 40,000 / plate under the following conditions: duplicate nitrocellulose filters (S&S) were hybridized overnight at 42.degree. C. in a solution containing 50% deionized formamide, 5.times.SSC (sodium chloride, sodium citrate), 0.1 mg / ml heat denatured salmon sperm DNA, 0.1...
example 3
Expression of P.sub.2U2 mRNA in Various Tissues and Cell Lines
Poly-(A)+ RNA was isolated from a variety of cell lines as described above. Five .mu.g of each sample was denatured, electrophoresed on a 1.2% formaldehyde agarose gel, and transferred to nylon membrane. Blots were probed with [.alpha.-.sup.32 P]dCTP(NEN) labeled insert, as described for the library screenings, and hybridized at 42.degree. C. overnight in the following solution: 5.times.SSPE, 1.times.Denhardt's, 50% formamide, 2% sodium dodecylsulfate, 0.1 mg / ml heat denatured salmon sperm DNA. Blots were washed twice at room temperature for 15 minutes in 0.05% SDS, 2.times.SSC and then at 50.degree. C. for 30 minutes in 0.1.times.SSC, 0.1% SDS and exposed at -70.degree. C. to Kodak XAR film for 48-72 hours with an intensifying screen. Northern blots containing poly-A+ RNA from human tissues were purchased from Clontech and hybridized, washed and exposed as described above. Hybridization of RNA tissue blots with the label...
example 4
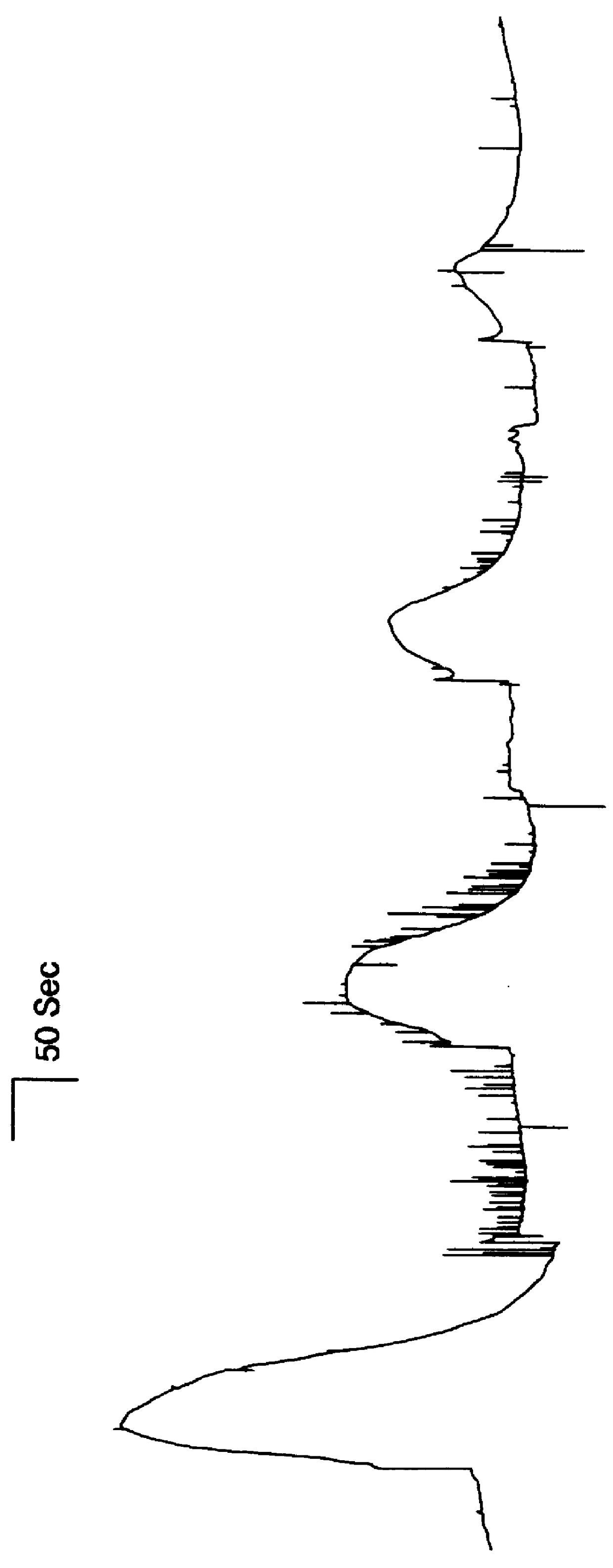
Demonstration of the Function of the Receptor in Oocytes
The native human receptor was produced in oocytes by cloning the 500 bp 5' truncation of the full-length kidney cDNA clone into the mammalian expression vector pcDNA3 (Invitrogen). Linearized DNA was used as a template for T7 polymerase (Ambion, Promega) for generation of capped in vitro transcribed mRNA following the supplier's specifications. Adult female Xenopus laevis were anesthetized in [0.015 g / l] 3-aminobenzoic acid ethyl ester for 10 minutes and 1 or 2 ovarian lobes were removed, followed by immediate suturing of the incisions. Oocytes were defolliculated at room temperature with collagenase (2 mg / ml) in Ca.sup.+2 -free medium (OR-2) for 1-2 hr. Oocytes were stored at 18.degree. C. in ND-96 (96 mM sodium chloride, 2 mM potassium chloride, 1.8 mM calcium chloride, 1 mM magnesium chloride, 5 mM HEPES (N[2-hydroxyethylpiperazine-N'[2-ethanesulfonic acid) with penicillin / streptomycin and injected with 50 nl RNA (1-2 .mu.g / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com