Cationic liposome delivery of taxanes to angiogenic blood vessels
a technology of cationic liposomes and taxanes, which is applied in the field of liposome delivery, can solve the problems of not being able to fully appreciate the specificity of cationic liposomes for preferential targeting angiogenic endothelial cells, and not being able to clearly determine, so as to enhance wound healing, inhibit angiogenesis, and promote angiogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Distribution of Cationic Liposomes in Normal Mice
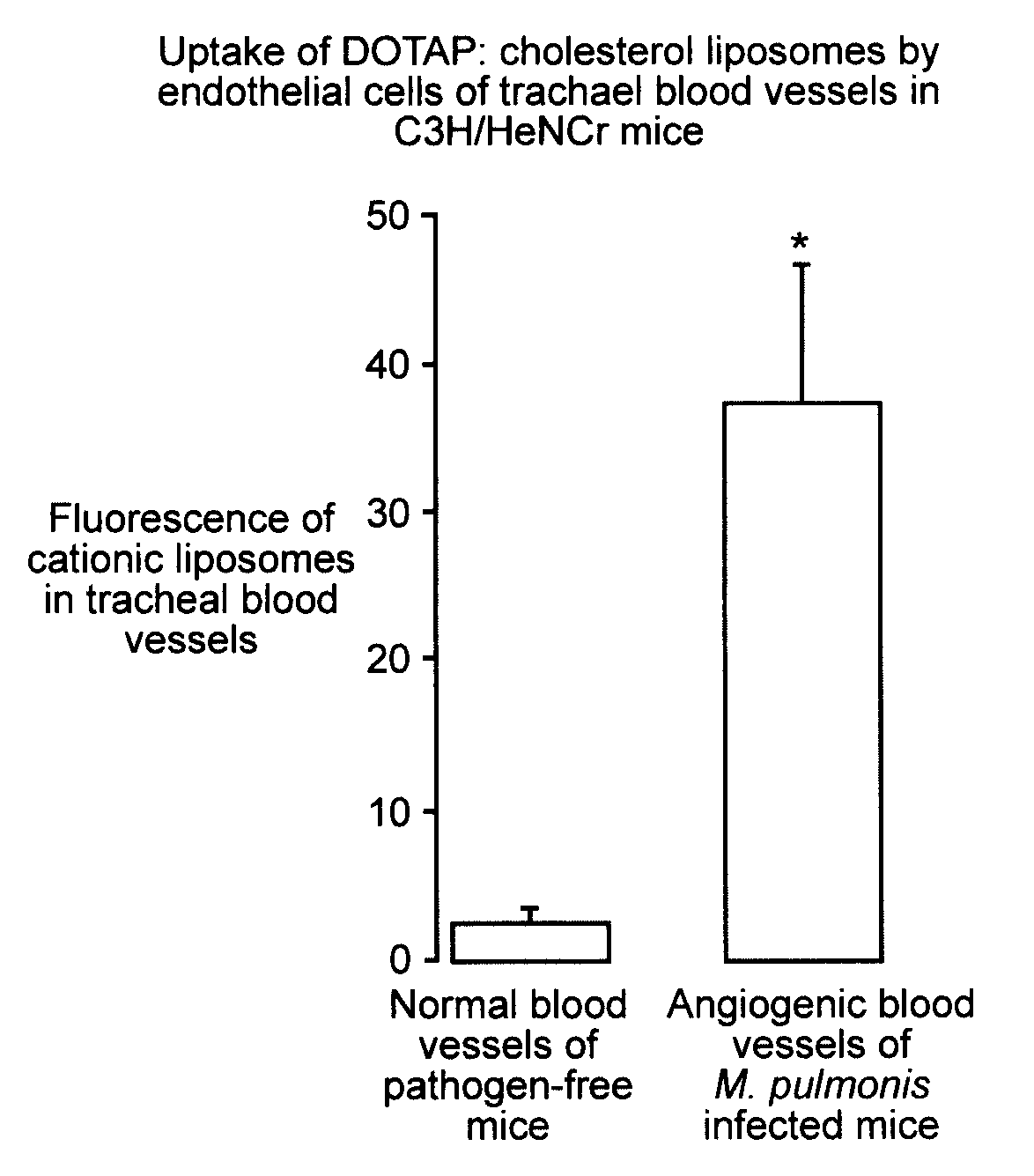
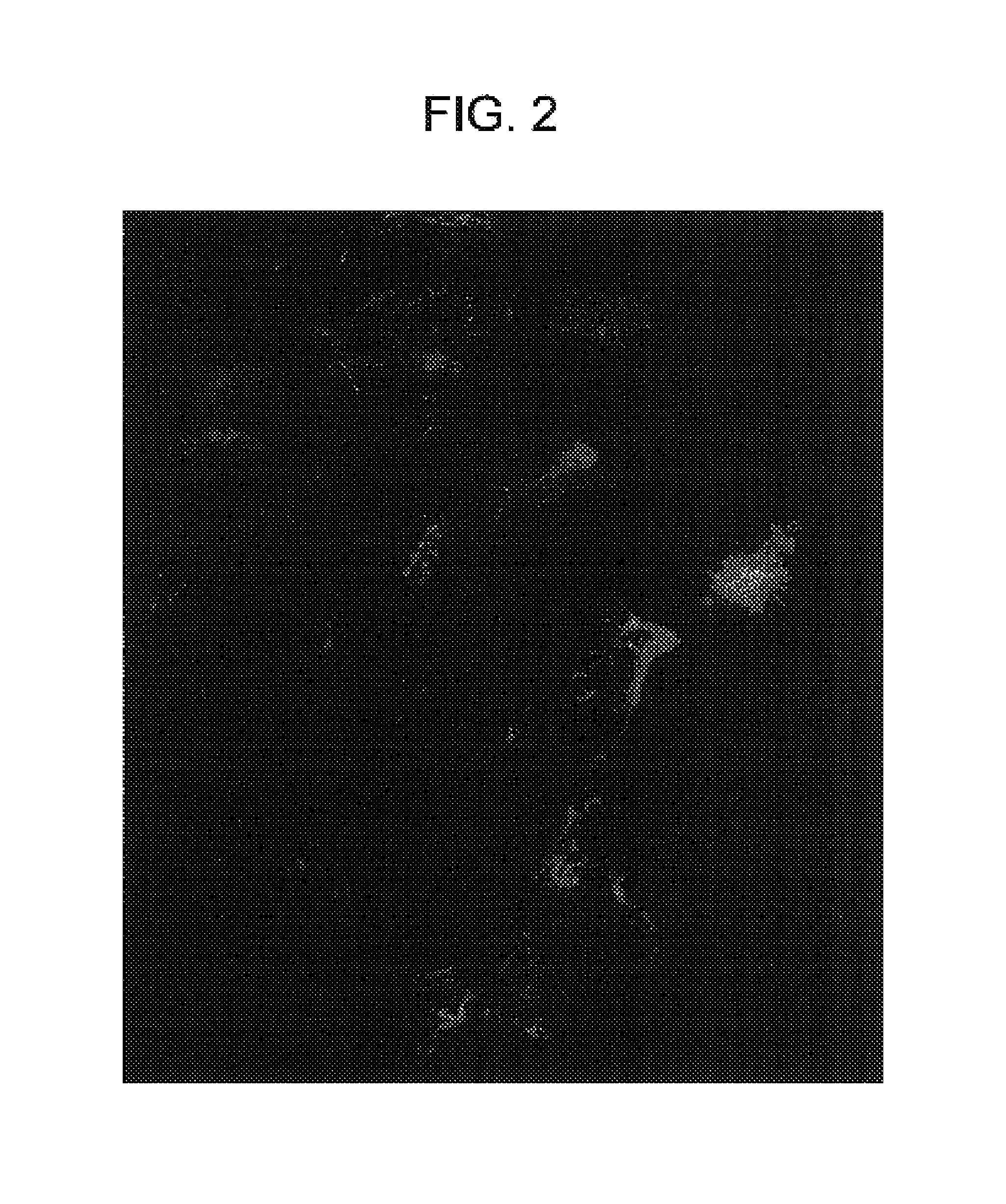
[0151]Liposomes and / or the plasmid DNA were labeled and the cellular distribution of the labeled complexes at various times after intravenous injection were determined. The experiments were performed on pathogen-free mice (20–25 g body weight) of both sexes.
[0152]Cationic small unilamellar vesicle liposomes were prepared from the cationic lipid DDAB or DOTAP and the neutral lipid DOPE or cholesterol, labeled with Texas Red or the red fluorescent carbocyanine dye DiI or CM-DiI, and in some cases complexed to plasmid DNA containing a reporter gene such as luciferase or β-galactosidase. Endothelial cells were labeled using the fluorescent plant lectin fluorescein-Lycopersicon esculentum. Monocyte / macrophages were labeled by using fluorescent beads (Duke, 500 nm). Cell nuclei were labeled with DAPI, YO-PRO, or Hoechst 33342 dye.
[0153]Fluorescent liposomes or liposome-DNA complexes containing 10–60 μg of DNA in up to 300 μl were injected i...
example 2
Uptake of DDAB:cholesterol Liposomes or Liposome-DNA Complexes in RIP-Tag5 Mice
[0163]The results of the experiments of Example 1 indicated that angiogenic blood vessels in ovarian follicles and corpora lutea avidly took up cationic liposomes and liposome-DNA complexes. Accordingly, an experiment was performed to determine whether endothelial cells of angiogenic blood vessels of tumors avidly take up cationic liposomes or liposome-DNA complexes.
[0164]The transgenic RIP-Tag5 model of tumors was used. see, Hanahan, (1985) Nature 315:115–22; and Hanahan and Folkman (1996) Cell 86: 353–64. In this model, designated RIP-Tag, the oncogene from the SV-40 virus, large T antigen (Tag), is driven by a region of the rat insulin promoter (RIP). When inserted into the murine genome, this construct induces the expression of the T antigen specifically in β-cells of pancreatic islets.
[0165]One important attribute of this model is that various stages of tumor development, and therefore various stages...
example 3
Uptake of DOTAP:cholesterol Liposomes-DNA Complexes in RIP-Tag2 Mice
[0169]Purpose: The fluorescence intensity of the liposome-DNA complexes had been increased by using Texas Red-DHPE in place of DiI; the method of preparing the pancreas of RIP-Tag2 mice for localizing sites of uptake of fluorescent cationic liposome-DNA complexes was improved; and the structure and function of the angiogenic blood vessels in pancreatic islet cell tumors in RIP-Tag2 mice had been studied. With these improvements experiments of the type described in Example 2 were carried out to determine how cationic liposomes and lipid-DNA complexes were taken up.
[0170]Methods: Cationic DOTAP:cholesterol small unilamellar vesicle liposomes, labeled with Texas Red-DHPE, were prepared. Liposome-DNA complexes were prepared at a total lipid:DNA ratio of 24:1 (nmoles / μg) in 5% glucose, using 60 μg of plasmid DNA in 300 μl Complexes (300 μl) were injected into tail veins of unanesthetized transgenic RIP1-Tag2 C57BL / 6 mice...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com