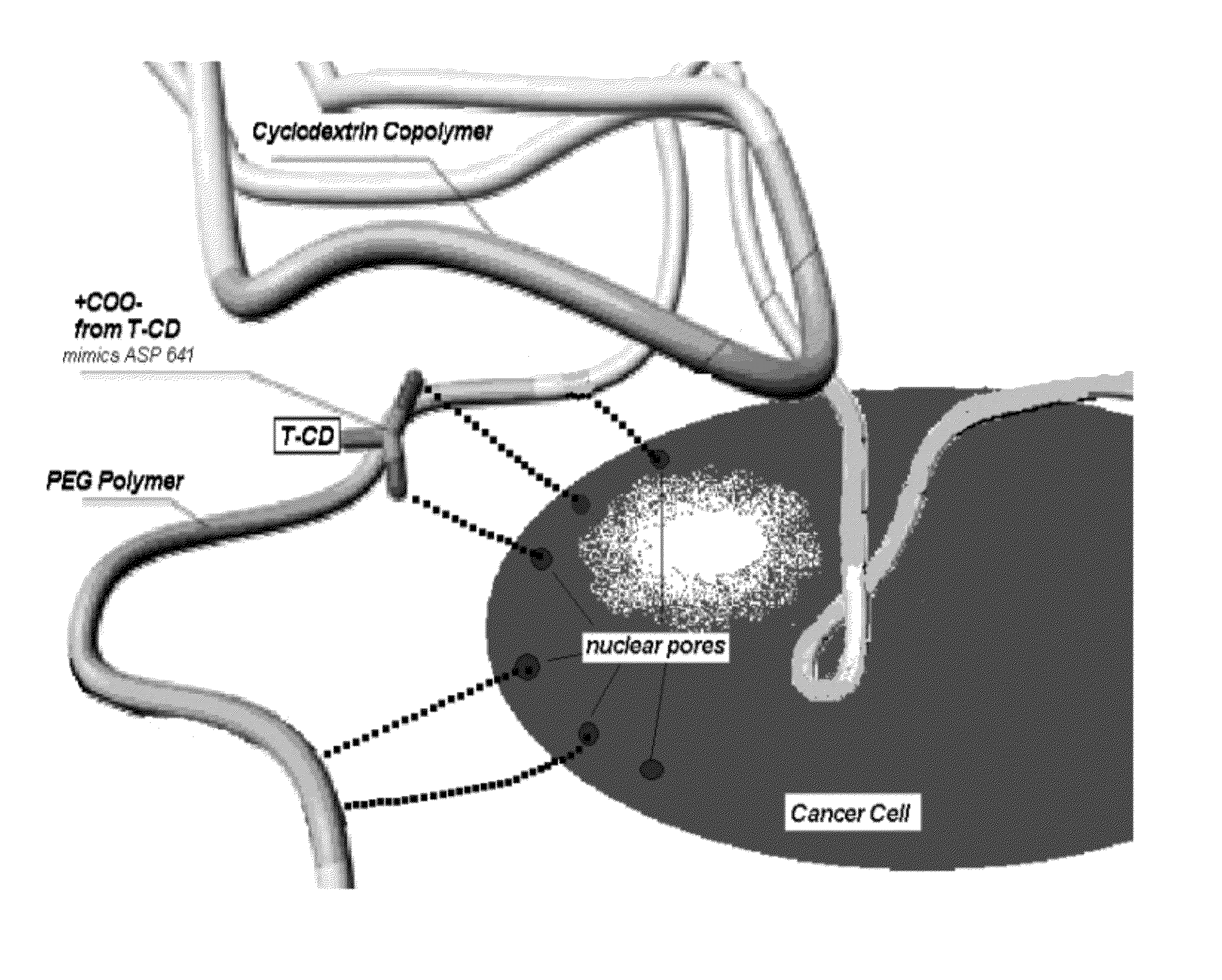
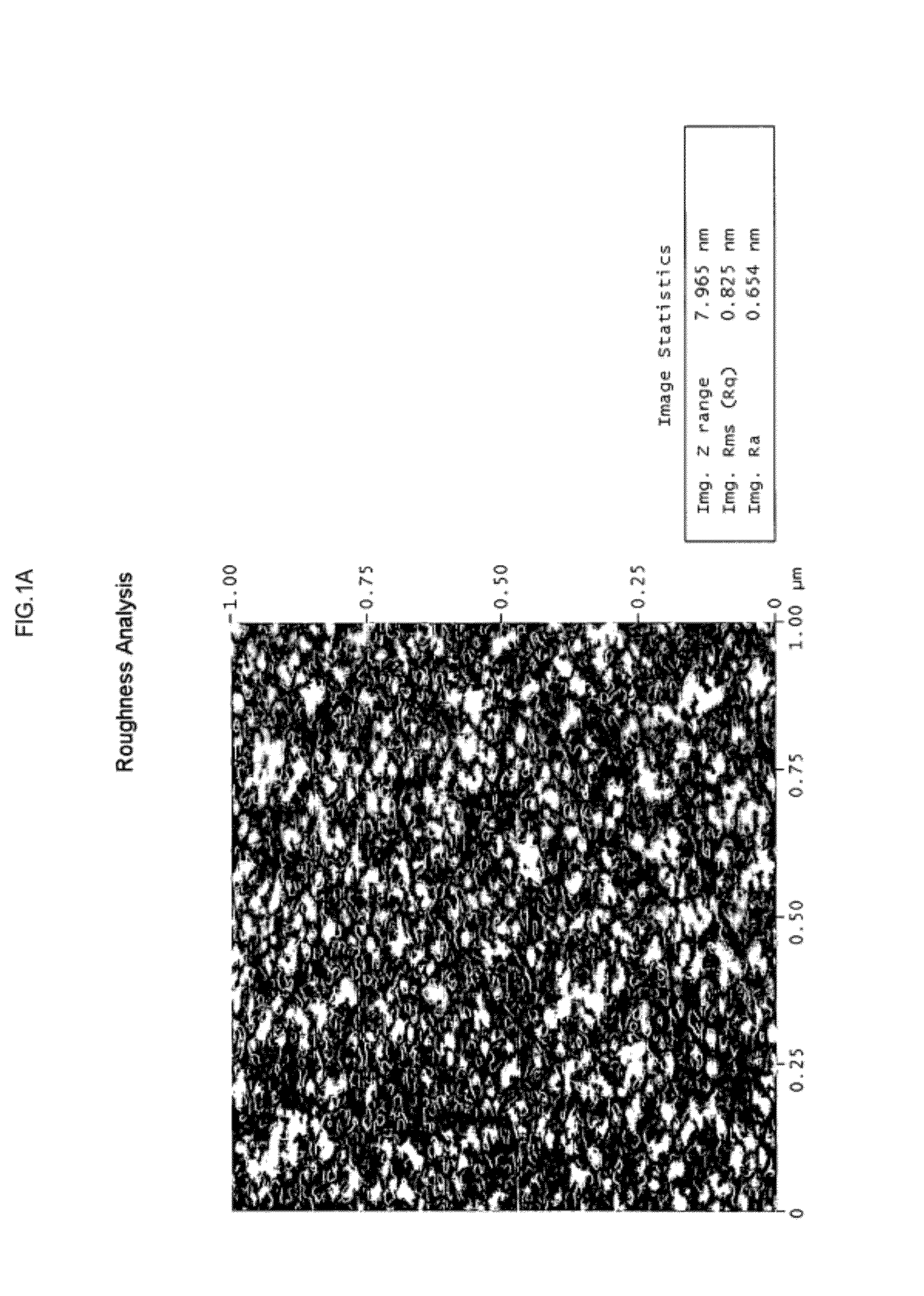
Nanopore array structured devices for biosensing and energy storage
a structured device and nanopore technology, applied in the field of biosensors, can solve the problems of insufficient robustness, limited development of glucose sensor technology, and insufficient development stage of technology, so as to avoid biofouling and denaturation, and improve the efficiency of the system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Constructing the Biosensor
[0064]Reagent grade poly (4-vinylpyridine) (PVP), polyethylene glycol diglycidyl ether (PEG), triacetyl-β-CD (T-β-CD), β-CD / epichlorohydrin, β-D-glucose were purchased from Aldrich-Sigma. The PVP was recrystallized in methanol. The biomimetic glucose enzyme, which is a biomimetic Histidine residue (His-516) receptor of glucose oxidase and mimics the active center of native glucose enzyme, named mM-β-DMCD was synthesized generally according to the published procedures (E. T. Chen and H. L. Pardue, Analytical applications of catalytic properties of modified-cyclodextrins. Anal. Chem. 65, 2563-2567, 1993, which is hereby incorporated by reference in its entirety as if set forth herein). U.S. Pat. No. 6,582,583 issued on Jun. 24, 2003 is also hereby incorporated by reference in its entirety as if set forth herein. Briefly, β-DMCD may be reacted first with sodium hydride in dry tetrahydrofuran under a nitrogen atmosphere at 35-38° C. for 10 hours. The solution i...
example 10
[0089]In addition to gold, glassy carbon can be used for construction of the biosensor of the present invention. The DET effect was observed and the irreversible peaks were also obtained.
[0090]The foregoing is considered as illustrative only of the principles of the invention. Further, since numerous modifications and changes will readily occur to those skilled in the art, it is not desired to limit the invention to the exact construction and operation shown and described, and, accordingly, all suitable modifications and equivalents may be resorted to, falling within the scope of the invention.
[Following Examples are for CIP Application]
example 11
Constructing the Biosensor
[0091]Reagent grade poly(4-vinylpyridine) (PVP), polyethylene glycol diglycidyl ether (PEG), triacetyl-β-CD (T-β-CD) and β-CD / epichlorohydrin were purchased from Aldrich-Sigma. PVP was purified before use. The biomimetic glucose enzyme, which is a biomimetic Histidine residue (His-516) receptor of glucose oxidase and mimics the active center of native glucose enzyme, named mM-β-DMCD was synthesized generally according to the published procedures (E. T. Chen and H. L. Pardue, Analytical applications of catalytic properties of modified-cyclodextrins. Anal. Chem. 65, 2563-2567, 1993, which is hereby incorporated by reference in its entirety as if set forth herein). U.S. Pat. No. 6,582,583 issued on Jun. 24, 2003 is also hereby incorporated by reference in its entirety as if set forth herein. Briefly, β-DMCD may be reacted first with sodium hydride in dry tetrahydrofuran under a nitrogen atmosphere at 35-38° C. for 10 hours. The solution is then cooled to 0° C....
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface smoothness | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com