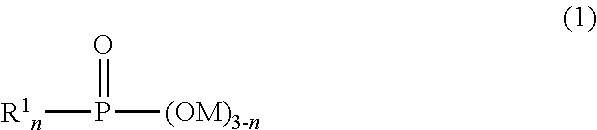Base metal pigment, aqueous base metal pigment dispersion liquid, and aqueous ink composition
a technology of base metal pigment and dispersion liquid, which is applied in the field aqueous base metal pigment dispersion liquid and aqueous ink composition, can solve the problems of difficult application of multi-product production, low on-demand properties, and difficulty in forming fine patterns or applying these methods to curved surfaces, etc., to achieve easy dissolution, improve water resistance of base metal pigment, and improve the dispersion stability of base metal pigmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
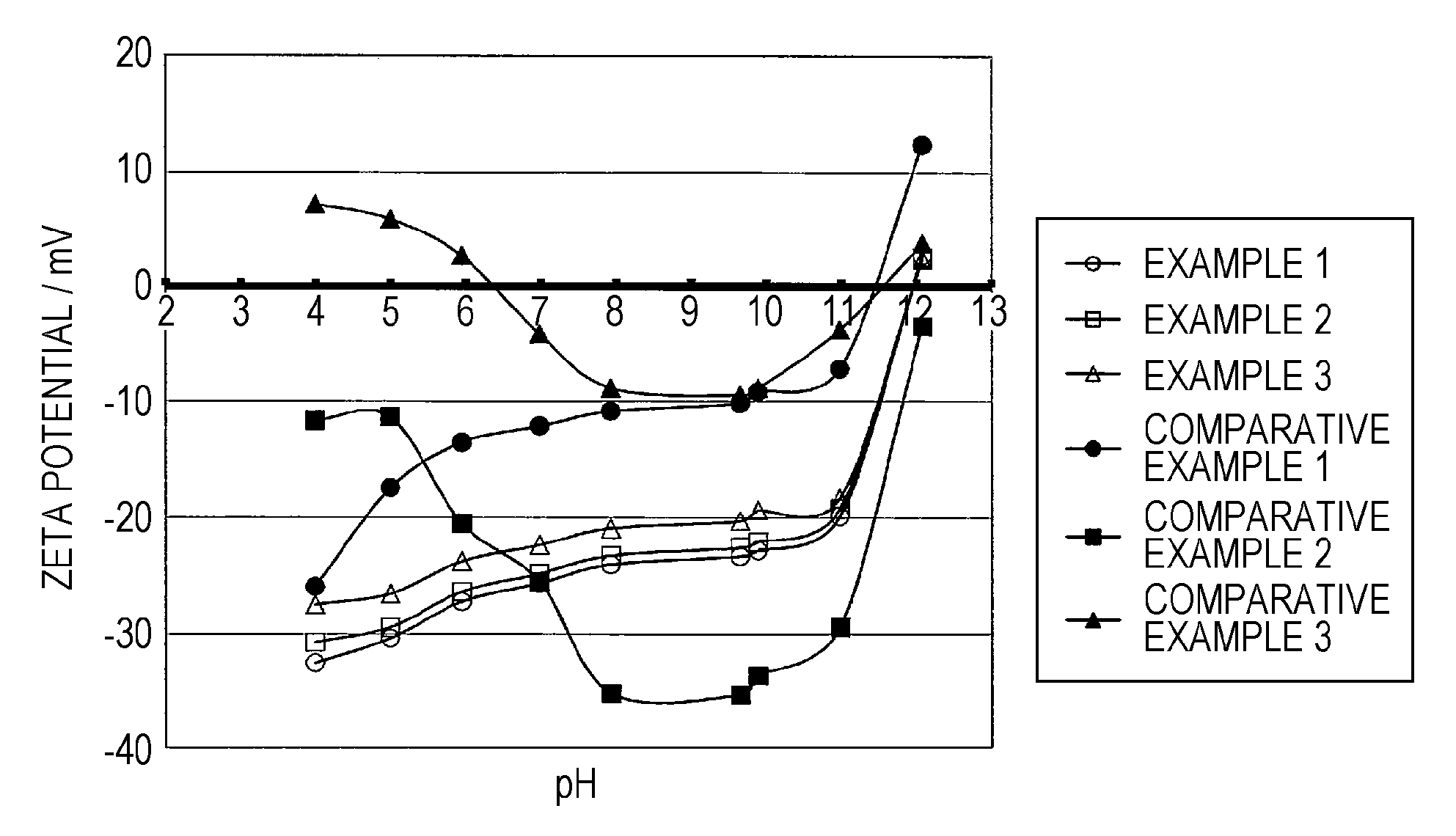
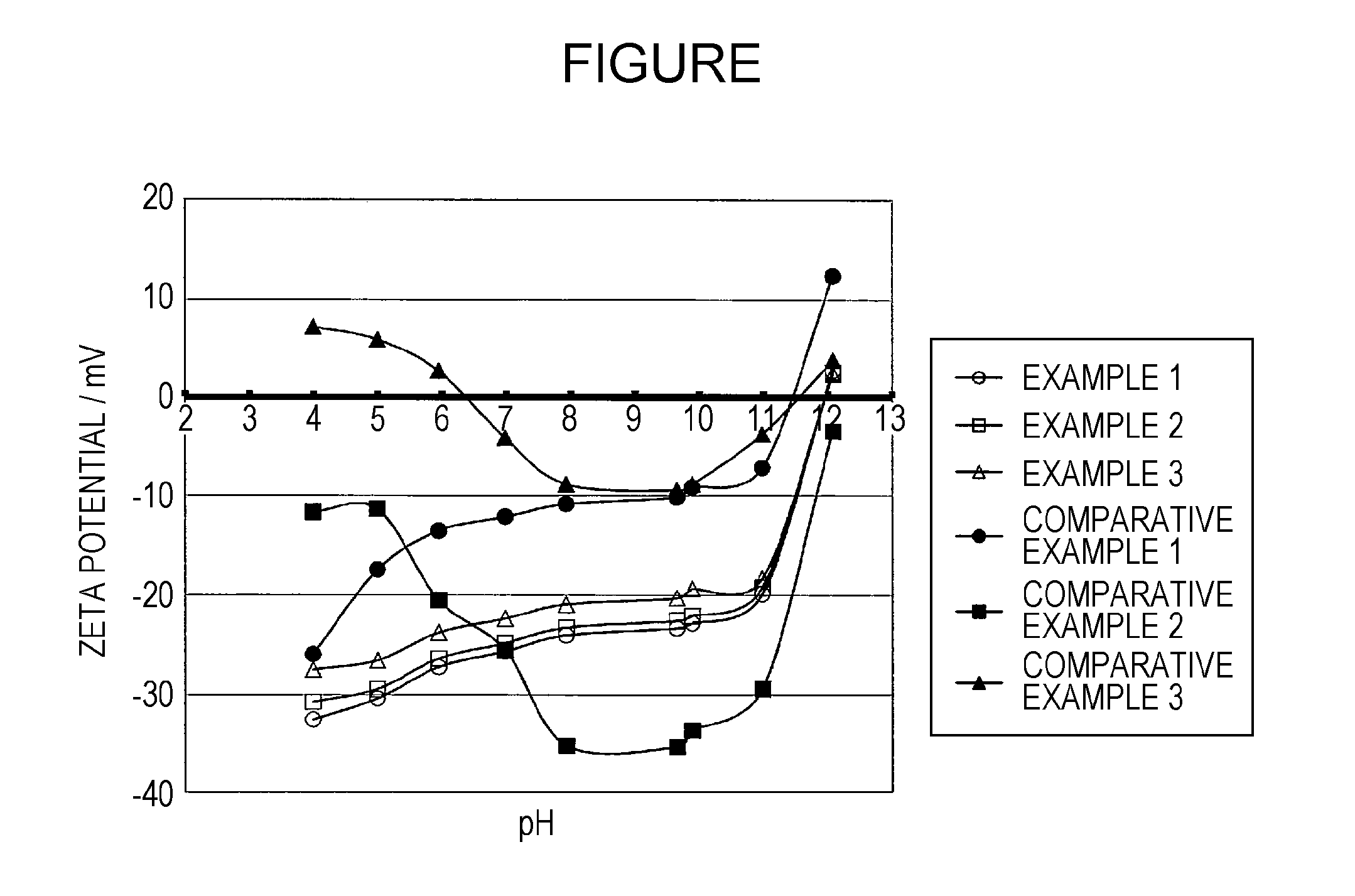
example 1
4.1. Example 1
4.1.1. Preparation of Aluminum Particle Dispersion Liquid
[0110]First, a polyethylene terephthalate-made film having a flat and smooth surface (surface roughness Ra: less than or equal to 0.02 μm) was prepared.
[0111]Subsequently, the entire one side of this film is coated with silicone oil. A film made of aluminum (hereinafter, simply referred to as “an aluminum film”) was formed on the surface side coated with this silicone oil using an evaporation method.
[0112]Subsequently, the film provided with the aluminum film was immersed in diethylene glycol diethyl ether, and was irradiated with ultrasonic waves to strip and pulverize the aluminum film from the film. Subsequently, this aluminum film was put into a homogenizer, and was pulverized for about 8 hours to obtain a dispersion liquid of scale-like aluminum particles (mother particles). The concentration of aluminum particles in this dispersion liquid was 10 mass %.
[0113]Subsequently, 100 parts by mass of diethylene gly...
examples 2 and 3
4.2. Examples 2 and 3
[0120]The aqueous ink compositions of Examples 2 and 3 were prepared and the measurement and evaluation thereof were conducted in the same manner as Example 1, except that the aqueous medium to be added at the time of preparing the aqueous ink composition was changed to those given in Table. The results thereof are given in Table.
PUM
| Property | Measurement | Unit |
|---|---|---|
| zeta potential | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com