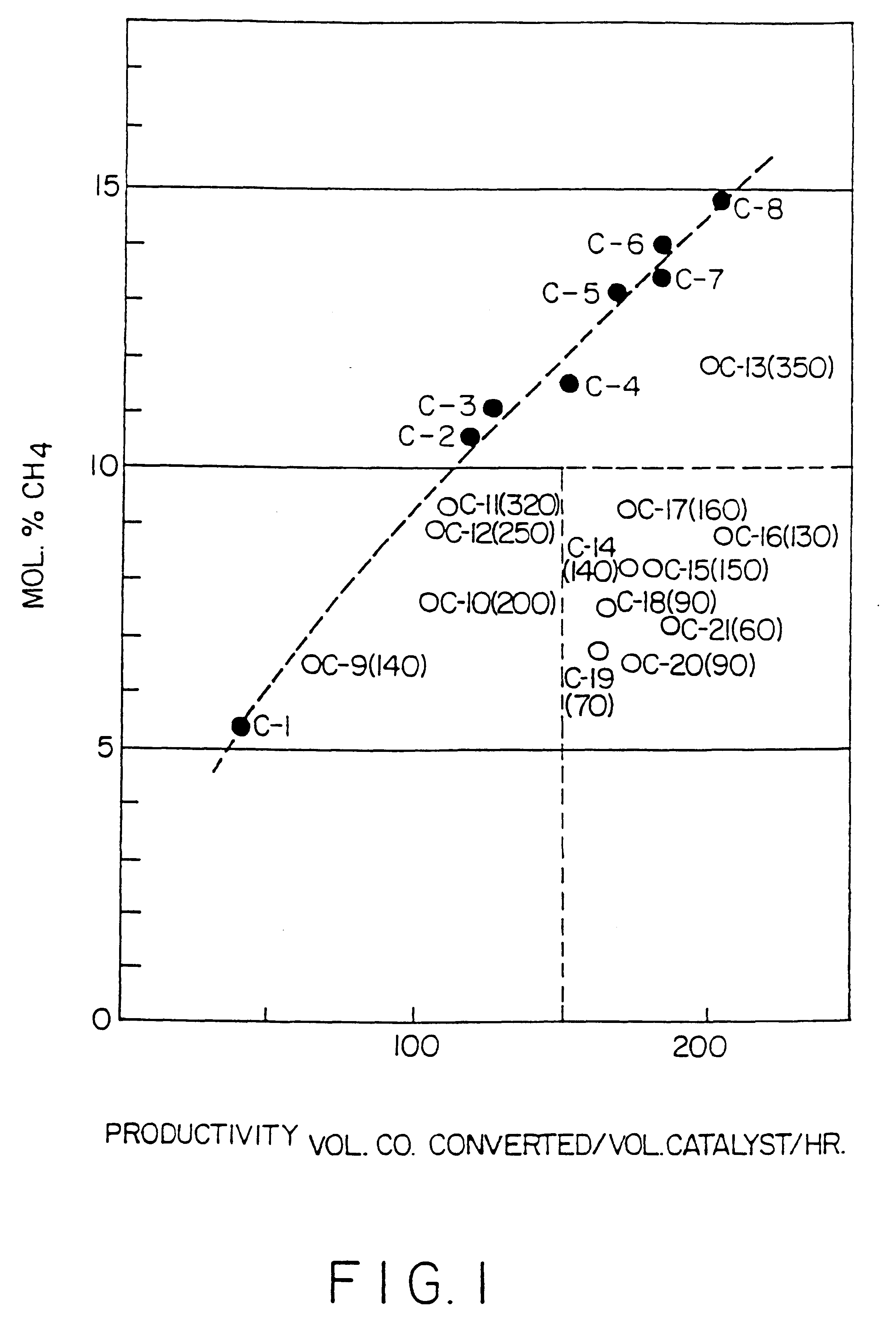
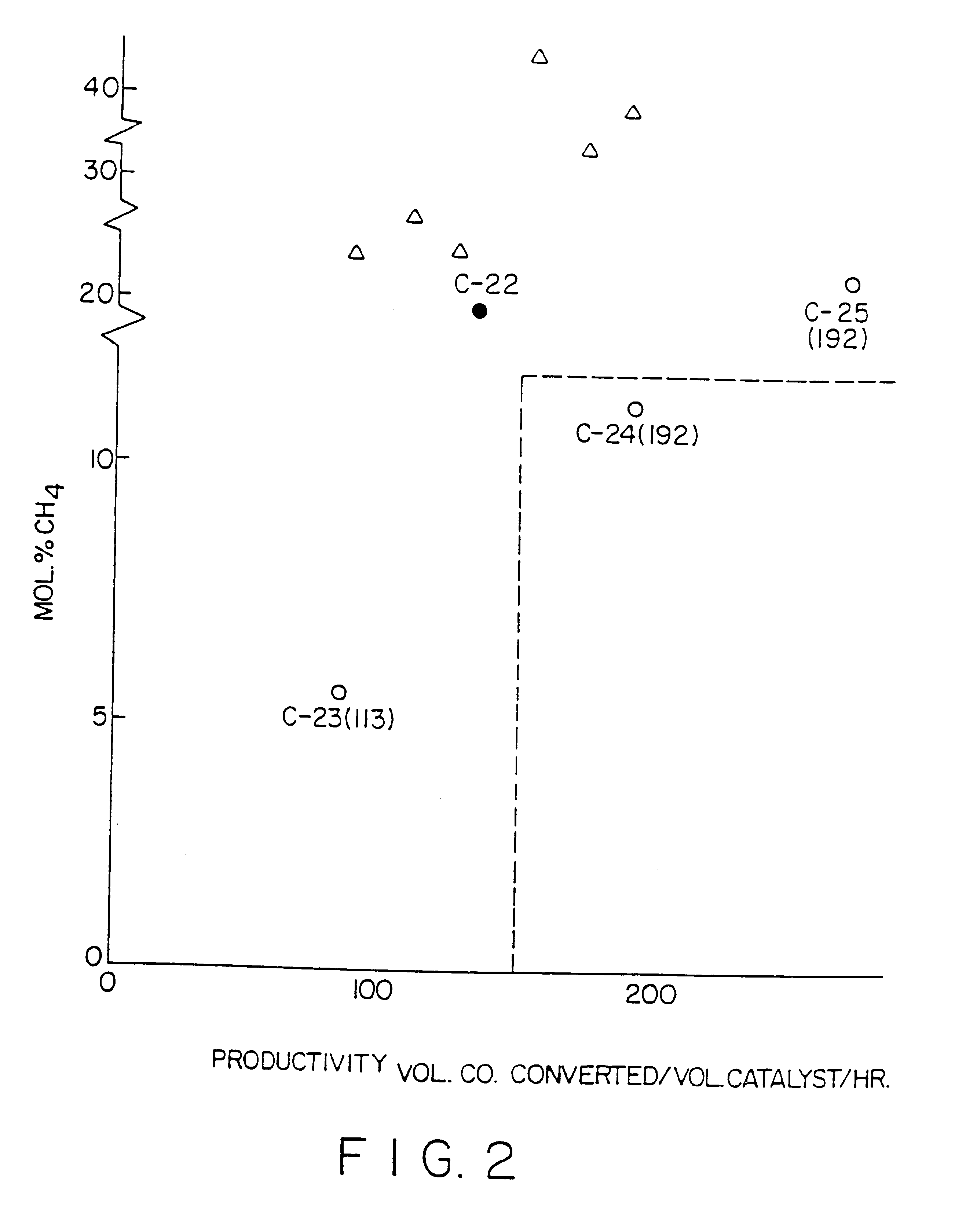
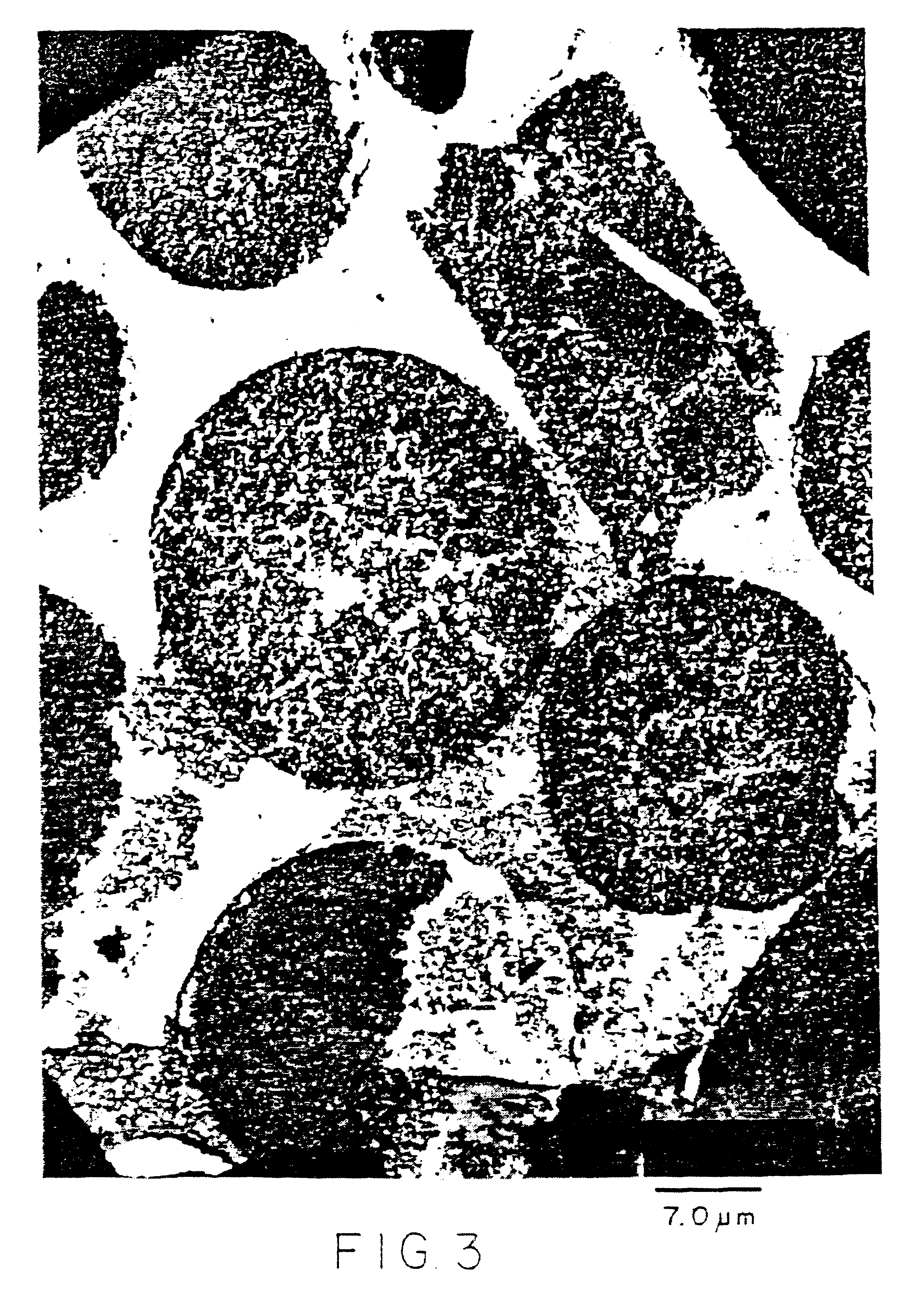Surface supported cobalt catalysts, process utilizing these catalysts for the preparation of hydrocarbons from synthesis gas and process for the preparation of said catalysts
a technology of cobalt catalysts and catalysts, which is applied in the field of surface supported cobalt catalysts, process utilizing these catalysts for the preparation of hydrocarbons from synthesis gas and process for the preparation of said catalysts. it can solve the problems of lessening the catalyst effectiveness, inability to easily move and transport to a more central location within the particle, and the effect of cobalt catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 9
About 14,500 lbs of finished catalyst was obtained by double impregnating a spray-dried support made from Degussa P-25 titania. The titania was spray-dried with an alumina sol binder. Product with an average particle size of about 45 microns was made in very high yield (97%). The support was then converted into the rutile form by calcination at high temperature in a 30".times.50" rotary calciner. Impregnation with an aqueous solution of cobalt nitrate and perrhenic acid was performed batch-wise in a 5 ft.sup.3 V-blender. The nitrate salt was decomposed by calcining the catalyst in the larger rotary at about 450.degree. C. A second pass through impregnation and calcination produced the finished catalyst.
High resolution imaging and EDS analysis of a cross-sectional view of the above catalyst is shown in FIG. 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| total pressure | aaaaa | aaaaa |
| total pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com