Synthetic biomaterial compound of calcium phosphate phases particularly adapted for supporting bone cell activity
a biomaterial and phase technology, applied in the direction of prosthesis, ceramics, other chemical processes, etc., can solve the problems of bloating performance in vivo, no prior composition developed, and no composition has been demonstrated to participate in the natural bone remodeling process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Ca—P Colloidal Suspension (Sol-Gel)
[0165]The following procedure is based on preparing sufficient sol-gel hydroxyapatite for manufacturing purposes. Solution A comprises a calcium nitrate tetrahydrate and Solution B comprises an ammonium dihydrogen orthophosphate (mono basic). Solution A is mixed with Solution B to produce the desired colloidal suspension. Solution A is prepared by adding 40 mls of doubly distilled water to 4.722 grams of calcium nitrate, Ca(NO3)2. The solution is stirred at moderate speed for sufficient time to dissolve all of the calcium nitrate which is normally in the range of 3 minutes. To this solution, 3 mls of ammonia hydroxide (NH4OH) is added and stirred for approximately another 3 minutes. To this solution is added 37 mls of double distilled water to provide a total solution volume of approximately 80 mls. The solution is stirred for another 7 minutes and covered. The pH of the solution is tested where a pH of about 11 is desired.
[0166]Solu...
example 2
Sintering of Ca—P Products
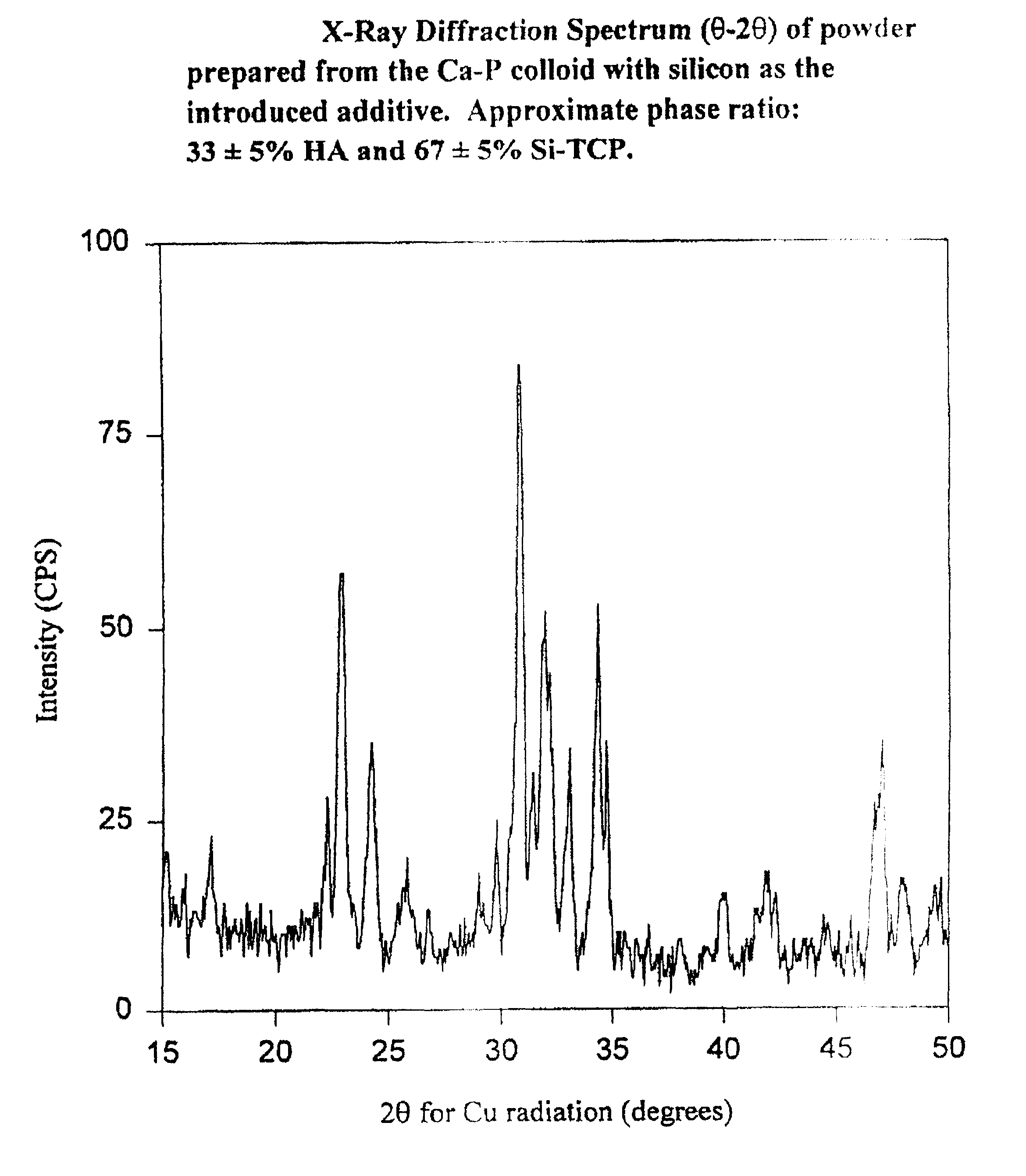
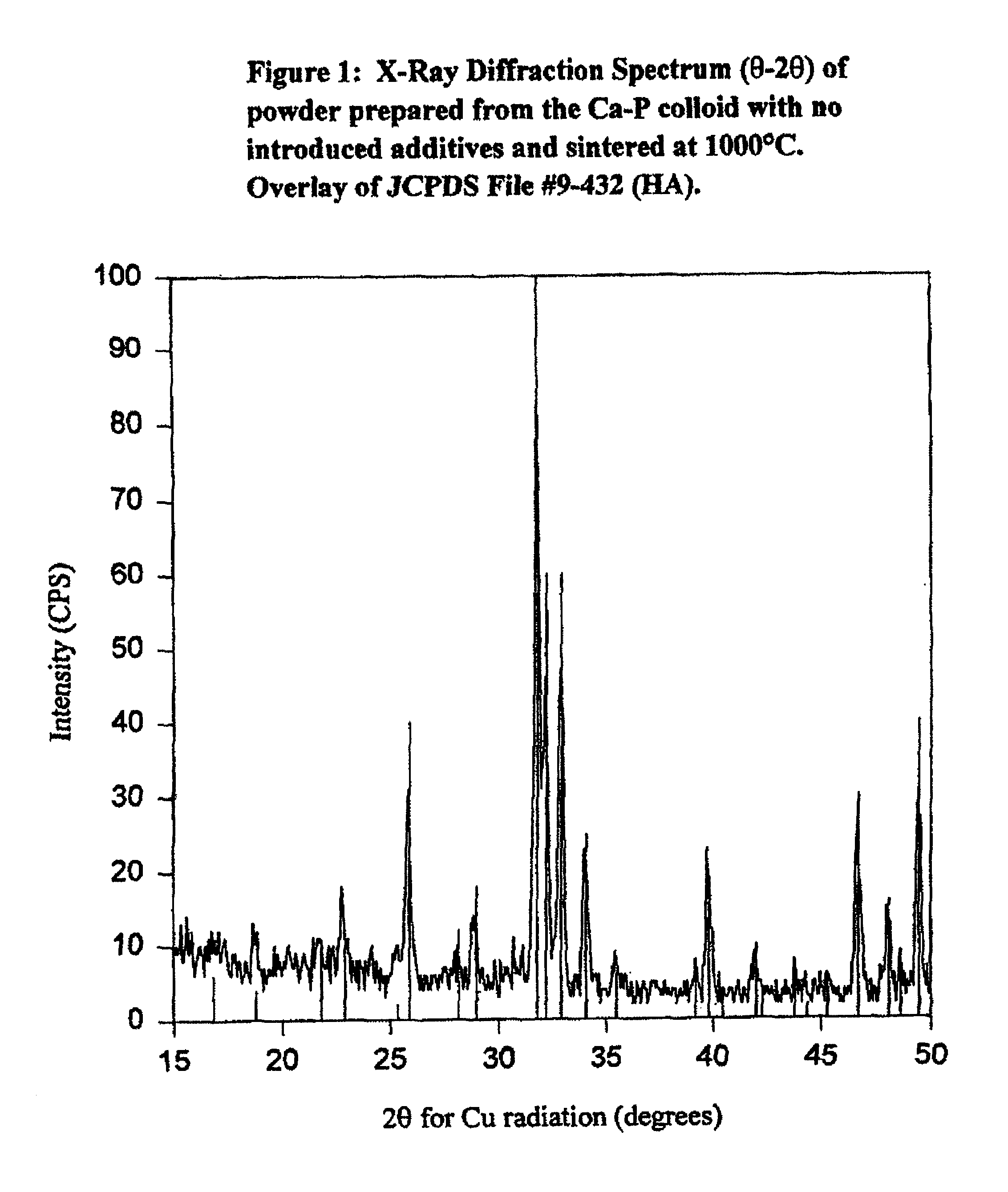
[0169]The following sintering process may be carried out in standard laboratory furnaces of various sizes, capable of operating at temperatures from ambient up to at least 1100° C., and designed to maintain accurate and stable internal temperatures, particularly between 800° C. and 1100° C., such as Lindberg models 51744 or 894-Blue M. The components prepared by any of the procedures described herein are carefully transferred onto a standard ceramic plate (as is common practice in the Lindberg oven). The ceramic plate is used as a carrier during the sintering process to facilitate easy loading and withdrawal of multiple substrates from the furnace. The furnace temperature is set to the temperature required to achieve the desired ratios of HA:SiTCP. Utilizing a programmable furnace such as the Lindberg model 894-Blue M, the furnace may be programmed to hold the desired temperature, which will normally be selected from the range 800° C. to 1100° C., for a max...
example 3
Preparation of Thin Films
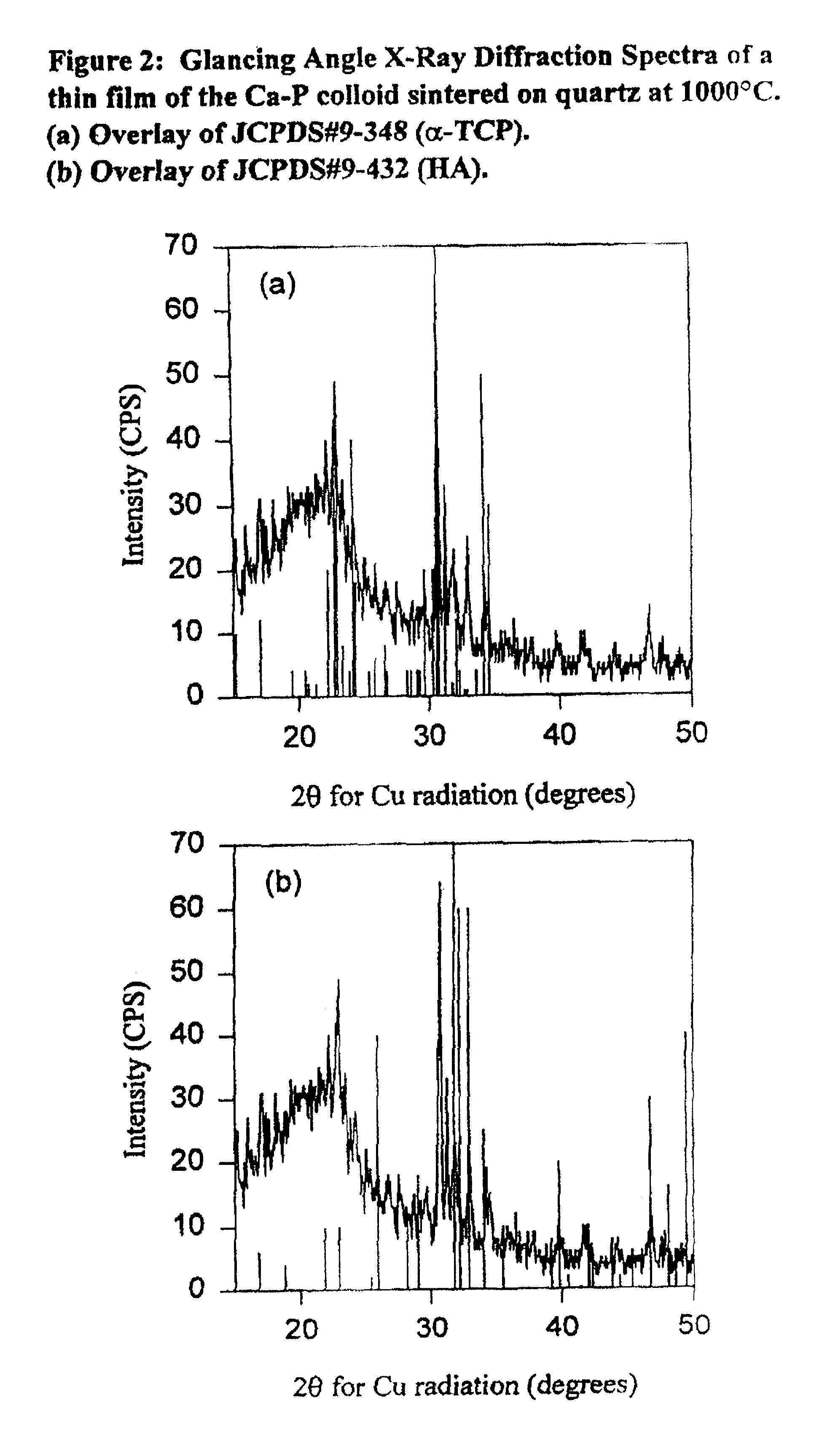
[0171]To create a thin film on a transparent substrate, quartz (amorphous silica) substrates were cleaned using water and chromic acid and subsequently dip coated in the colloidal suspension of Example 1. The substrate needs to be thoroughly cleaned to ensure satisfactory film coverage. In the case of quartz substrates, cleaning is achieved by placing the discs in a glass beaker and supplying chromic acid cleaning solution to the glass beaker to cover all discs. The beaker is then covered. The discs are then sonicated in a water bath for 1 hour. The acid is washed away using tap water for 20 minutes. The residual tap water is removed by three changes of doubly distilled water. After the final change of double distilled water, every single disc is dried with lint-free towel and inspected for flaws in the quartz surface. Any residual particulate on the surface is removed as needed with compressed nitrogen or air. The discs are stored in covered trays in an ase...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| dwell time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com