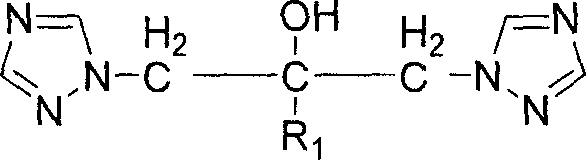Bi-triazole compound, preparation method and application thereof
A compound, bitriazole technology, applied in the field of bitriazole compound and its preparation, to achieve the effect of simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
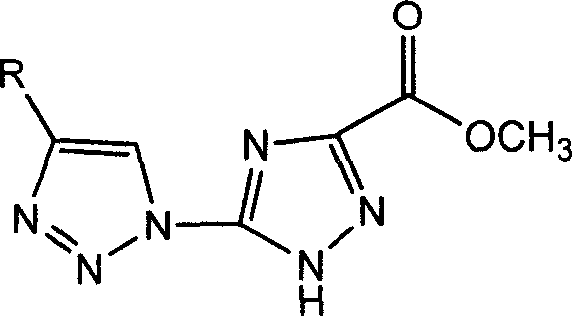
[0032] 5-Azido-1,2,4-triazole-3-acylmethyl ester (50 mg, 0.297 mmol) was placed in a two-necked flask, vacuumized on an oil pump for 20 minutes, and then 10 mL of a mixed solvent (THF / H 2 O=1 / 4), a colorless and transparent solution was obtained. Then, propynyl acetate (35 mg, 0.36 mmol) was added under the protection of Ar gas. Separately, copper sulfate pentahydrate (11.8 mg, 0.047 mmol) and sodium ascorbate (29.5 mg, 0.148 mmol) were dissolved in 200 μL of water, and then injected into the above reaction system. After reacting at 80° C. for 1 hour, the reaction was stopped. At this time, the reaction system was a yellow turbid liquid. After the solvent was distilled off under reduced pressure, a yellow solid was obtained. The product was separated by column chromatography (CH 2 Cl 2 / MeOH=30 / 1), the obtained product (1a) was 73.1 mg of white solid after vacuum drying, and the yield was 92.4%.
[0033] 1 H NMR (300MHz, DMSO-d 6 ): δ8.79(s, 1H), 5.22(s, 2H), 3.96(s, 3H)...
Embodiment 2
[0035] 5-azido-1,2,4-triazole-3-acylmethyl ester (50mg, 0.297mmol) was placed in a two-necked flask, vacuumized on an oil pump for 20 minutes, and then 5mL of a mixed solvent (THF / H 2 O=1 / 2), the raw materials were dissolved to obtain a colorless and transparent solution. Then p-pentanephenylacetylene (50mg, 0.297mmol) was added under the protection of Ar gas. Separately, copper sulfate pentahydrate (11.8 mg, 0.047 mmol) and sodium ascorbate (29.5 mg, 0.148 mmol) were dissolved in 200 μL of water, and then injected into the above reaction system. After reacting at 60° C. for 2 hours, the reaction was stopped. At this time, the reaction system was a brownish-yellow turbid liquid. After directly distilling off the solvent under reduced pressure, a brownish-yellow solid was obtained. The product was separated by column chromatography (CH 2 Cl 2 / MeOH=30 / 1), the obtained product (1b) was 82.2 mg of white solid after vacuum drying, and the yield was 81.2%.
[0036] 1 H NMR (30...
Embodiment 3
[0038] Put 5-azido-1,2,4-triazole-3-acylmethyl ester (50 mg, 0.297 mmol) in a two-necked flask, vacuumize it on an oil pump for 20 minutes, then add 12 mL of tetrahydrofuran, the raw material is dissolved to obtain a colorless Clear solution. Then cyclohexanol alkyne (54.7 mg, 0.44 mmol) was added under the protection of Ar gas. Separately, copper sulfate pentahydrate (11.8 mg, 0.047 mmol) and sodium ascorbate (29.5 mg, 0.148 mmol) were dissolved in 200 μL of water, and then injected into the above reaction system. After reacting at 90° C. for 2 hours, the reaction was stopped. At this time, the reaction system was a light yellow turbid liquid. After the solvent was distilled off under reduced pressure, a yellow solid was obtained. The product was separated by column chromatography (CH 2 Cl 2 / MeOH=30 / 1), the obtained product (1c) was 67.5 mg of white solid after vacuum drying, and the yield was 77.7%.
[0039] 1 H NMR (300MHz, DMSO-d 6 ): δ8.41(s, 1H), 5.07(br s, 1H), 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com