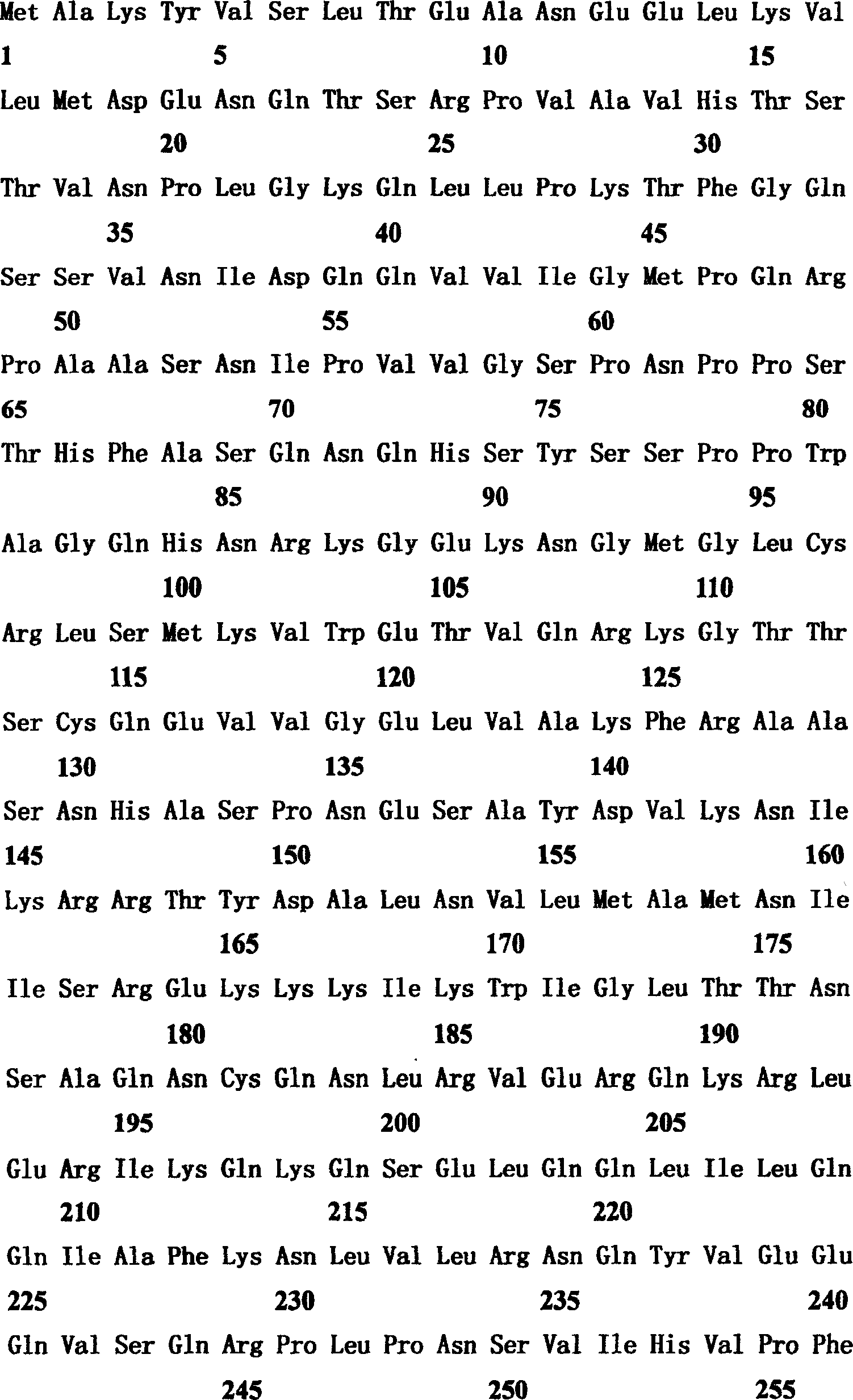Idiosyncratic antigen protein, and antigen peptide of liver cancer orchis pellet
A protein-specific technology, applied in the direction of peptide/protein components, specific peptides, peptides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1. Gene Analysis
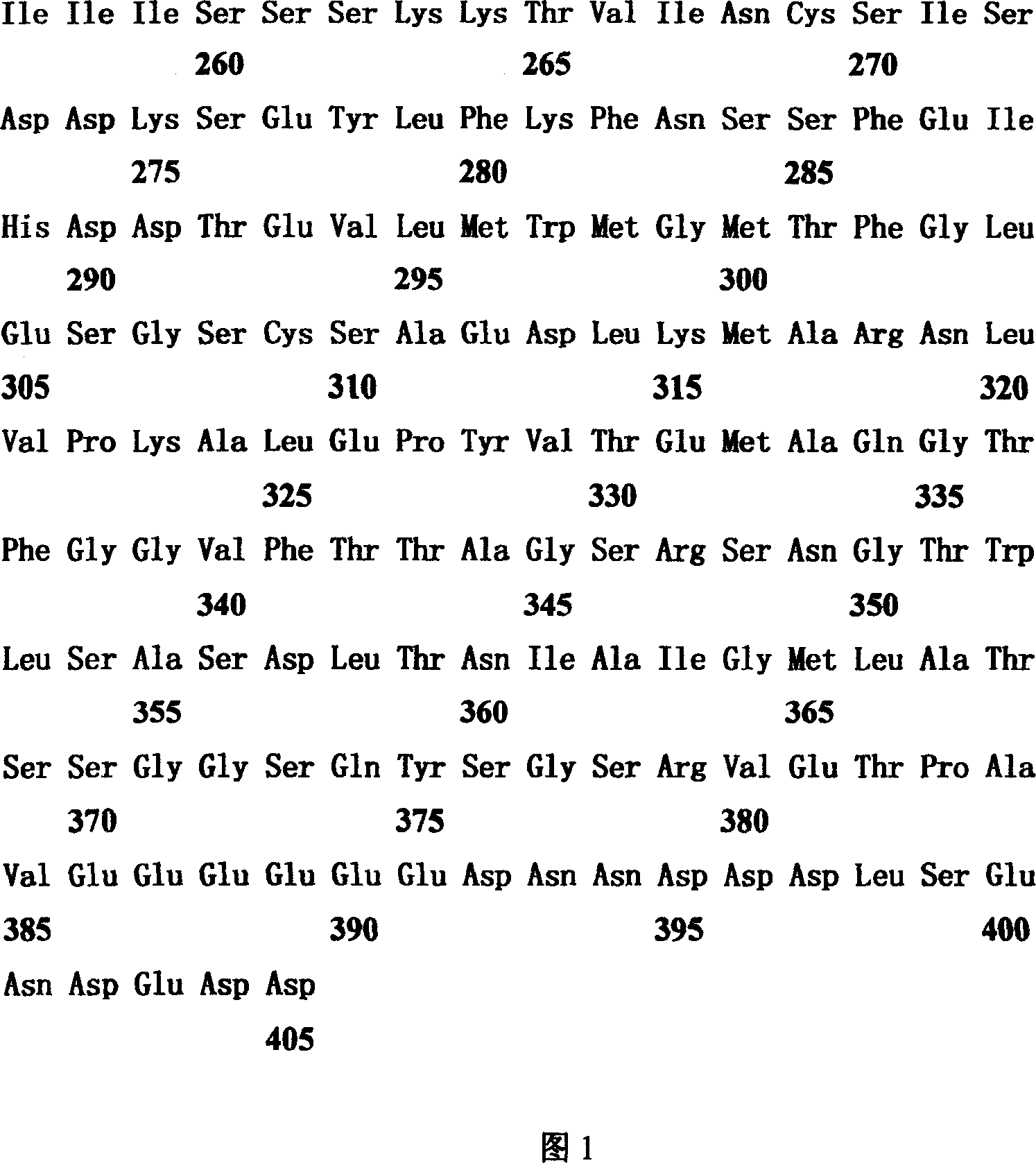
[0034] TFDP-3 (HCA661) is a new gene screened from the patient's liver cancer tissue with serum by SEREX method. According to bioinformatics analysis, the TFDP-3 (HCA661) gene is located on the X chromosome of the human gene and is a gene consisting of a single exon. The size of the transcript is 1,680bp, the coding frame is 1,218bp, the start and end points are 90-1,307, and it encodes a protein of 405 amino acids (as shown in Figure 1). This protein is a new member of the DP family of transcription factors, and has a high degree of similarity (75.2% amino acid identity; 82.0% amino acid similarity) to its DP family homologue TFDP-1. The comparative analysis of the amino acid sequences of the two is shown in Figure 3. Similar to TFDP-1, the amino acid sequence of TFDP-3 includes an evolutionarily conserved DNA binding domain (AA108-AA191) and an evolutionarily conserved heterodimerization structure domain (AA192-AA264), and, in the DNA-bind...
Embodiment 2
[0035] Example 2. TFDP-3 (HCA661) mRNA Northern blot analysis
[0036] For Northem blot analysis, total RNA was extracted from HCC specimens and corresponding noncancerous tissue specimens as well as testicular tissue. RNA integrity was analyzed by electrophoresis. During electrophoresis, the loading amount of total RNA per well was 30 mg, and then transferred to the membrane. with specificity 32 The P-labeled cDNA probe was hybridized to nitrocellulose membrane overnight at 65°C. Wash three times with 0.1×SSC / 0.1% SDS solution, each time for 30 minutes, and perform autoradiography at -70°C. The size of the full-length mRNA is compared with the degree of migration of 28S and 18S ribosomal RNA.
[0037] Due to the high similarity of nucleotide sequences, the results of Northern blot hybridization (as shown in Figure 4) showed that two strong bands appeared in the RNA of the testis tissue, which were ~1.7kb and 2.1kb respectively. Only a ~1.7 kb band appeared in the RNA of ...
Embodiment 3
[0038] Example 3. Expression distribution of TFDP-3 (HCA661) in HCC and adjacent non-cancerous tissue samples
[0039] The expression profile of TFDP-3 (HCA661) mRNA was analyzed by RT-PCR, and the results are listed in Table 1. Tissues included 16 normal tissue cDNAs purchased from Clontech: brain, heart, kidney, liver, lung, pancreas, placenta, skeletal muscle, colon, ovary, peripheral blood leukocytes, prostate, small intestine, spleen, testis, and thymus, from HCC tissue The total RNA extracted from the matched adjacent non-cancerous tissue was reverse transcribed into cDNA. The TFDP-3(HCA661) fragment was amplified using specific primer pairs. Glyceraldehyde 3-phosphate dehydrogenase (G3PDH) mRNA was used as an internal reference. The primers used are as follows: TFDP-3, forward, 5'-TACACT CGG CCT GGA AGA ATT G-3'; reverse, 5'-TCT TCC TCC TCG ACT GCT G-3', size, 1,244 base pairs (bp).
[0040] Among the 16 kinds of normal tissues, only the testis detected the high leve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com