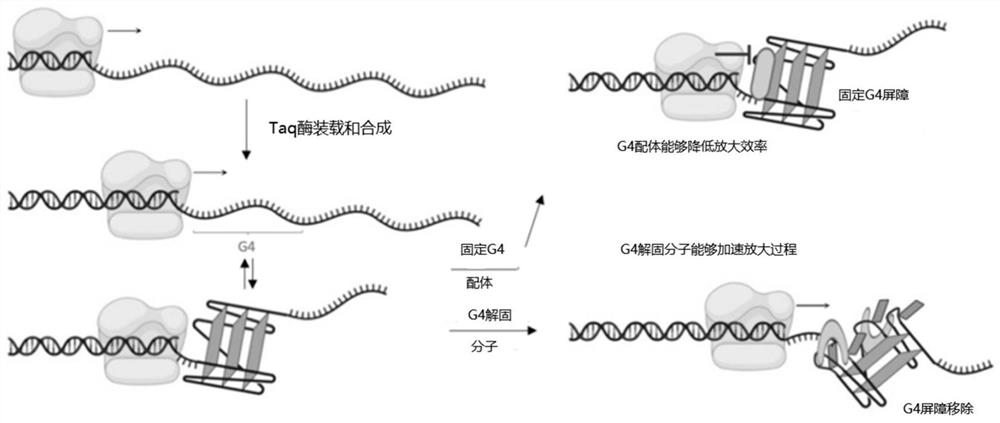
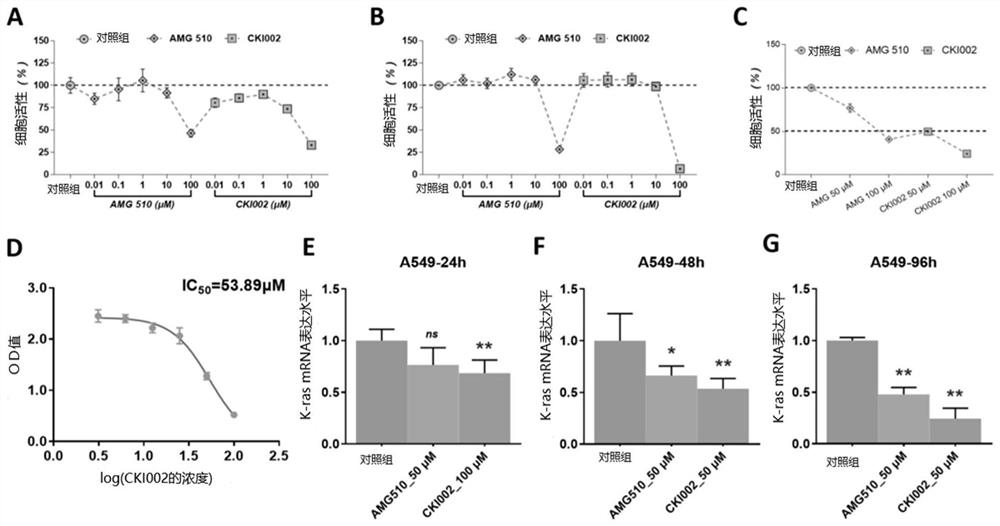
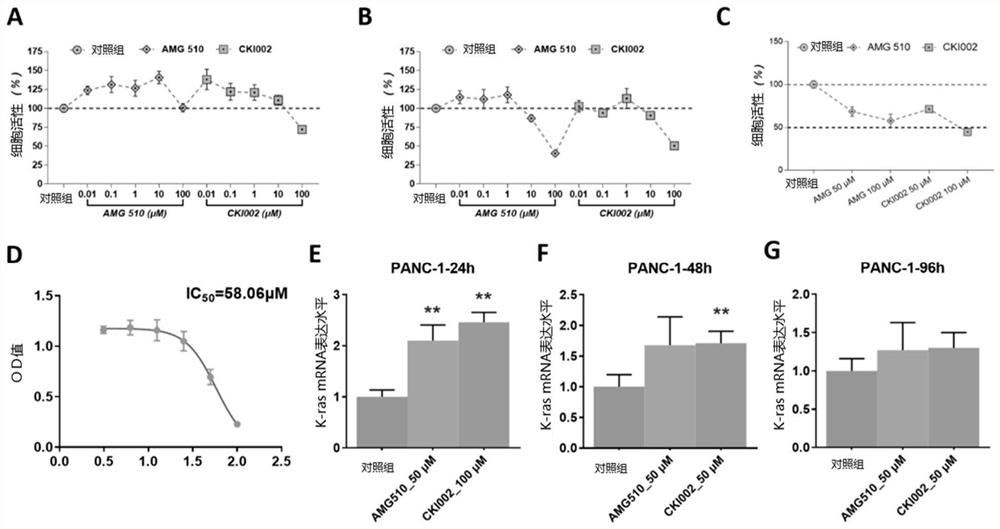
Amino steroid compound as well as preparation method and application thereof
A steroid compound and compound technology, applied in the field of chemistry, can solve the problems of loss of K-ras protein inhibitory effect and the inability of small molecule drugs to bind, and achieve the effect of promoting cancer cell death, tight binding and excellent inhibition.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] The compound having the following structure IV of Example 1 of the present application can be prepared by the following two process routes.
[0081]
[0082] Process route one:
[0083] (1.1) Dissolve quinolinic acid (10g, 57.7mmol) in 200ml of anhydrous DMF (N,N-dimethylformamide), add PyBOP (Benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphoniumhexafluorophosphate, hexafluorophosphate) Benzotriazol-1-yl-oxytripyrrolidinophosphorus fluorophosphate, 36g, 69.2mmol) and HOBt (N-hydroxybenzotriazole, 1-hydroxybenzotriazole, 9.43g, 69.2mmol) were added after stirring for 5min Proline tert-butyrate, followed by DIPEA (N,N-diisopropylethylamine, 40 mL, 0.22 mol). The above solution was stirred at room temperature and reacted overnight for 12 h, then the reaction solution was poured into 1 L of water, extracted three times with ethyl acetate, dried over sodium sulfate, and evaporated to obtain N-(quinolinic acid-2-carboxylate)-L-pro Acid tert-butyrate (compound 9) 13.1 g.
...
Embodiment 2
[0100] The compound having the following structure V of Example 2 of the present application can be prepared through the following two process routes.
[0101]
[0102] Process route one:
(2.1)
[0103] Starting from compound (3) (2β-piperazinyl-3α-hydroxy-5α-steroid-17-one), this compound (3) is combined with 1-(quinoline-2-ester group by acylation reaction )-L-proline (compound 10) reaction into which a 1-(quinolin-2-yl-carbonyl)-L-proline moiety was introduced to yield compound D having the following structural formula
[0104]
[0105] (2.2) 313 mg of compound D, 447 mg of copper bromide and 20 ml of methanol were put into the reaction flask, and the temperature was raised to 80° C. for reflux reaction for 15 hours. The reaction solution was cooled to room temperature, water and dichloromethane were added, and the mixture was stirred for ten minutes to separate the layers. The organic phase was washed once with saturated brine, and concentrated to dryness under r...
Embodiment 3
[0112] The compound having the following structure VI of Example 3 of the present application can be prepared by the following procedure.
[0113]
[0114] The compound of structure VI above was prepared in the same manner as in Example 1, except that in step (1.6), trimethylsilyl acetylene was replaced with trimethylsilyl chloroacetylene, and since the halogen had been attached onto acetylene, so the halogenation process in step (1.8) is omitted here. Compound VI thus obtained was characterized as follows.
[0115] tested, 1 H NMR (400MHz, CDCl 3 )δ8.21(dd,J=12.3,8.6Hz,1H),8.13-7.92(m,2H),7.85-7.79(m,1H),7.72(m,1H),7.62-7.52(m,1H) ,5.86 and 5.11(m,1H),4.23–3.11(m,8H),2.77–2.58(m,2H),2.51–2.04(m,5H),2.01–0.98(m,22H),0.86(2s, 3H), 0.82 (2s, 3H), 0.76–0.64 (m, 1H).
[0116] Mass Spectrometry (MS, EI), C 40 H 51 ClN 4 O 4 , 687.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com