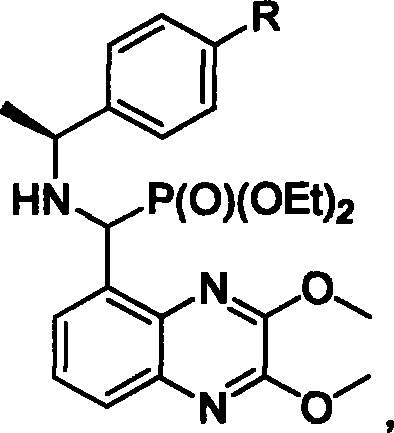
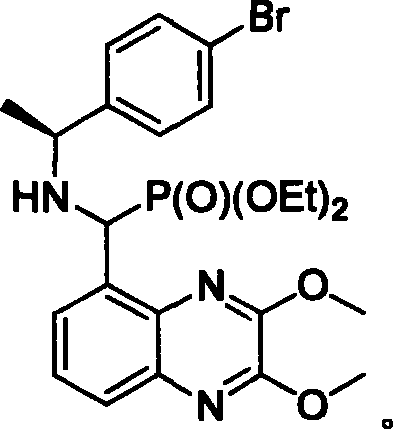
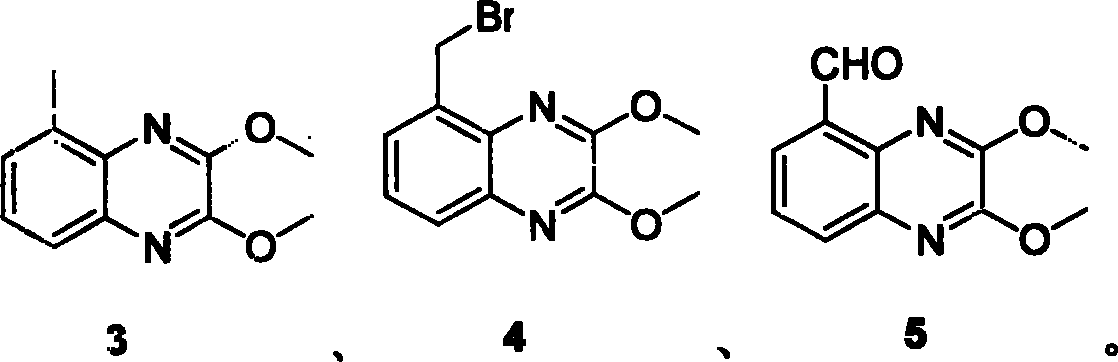
NMDA receptor antagonist intermediate, its synthesis and use
A technology of receptor antagonists and synthetic methods, applied in the field of intermediates of receptor antagonists, to achieve the effect of short synthetic route and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033]Compound 1 (8g, 67mmol) and oxalic acid (10g, 68mmol) were dissolved in hydrochloric acid solution (4N, 450ml), and then heated to reflux for 6 hours. After the reaction, cool naturally, adjust the PH value to be neutral, then filter, dissolve the brown solid with ether, wash with water, wash with saturated sodium chloride, dry over anhydrous sodium sulfate, filter, concentrate to obtain a brown solid, and dry the solid Weighed 11.6 g, 99% yield.
[0034] 1 HNMR (300MHz, CDCl 3 ): δ11.90 (1H, s), 11.21 (1H, S), 6.98-6.94 (3H, m), 2.33 (3H, s) ppm.
Embodiment 2
[0036]
[0037] Compound 2 (11.6g, 65.9mmol) was dissolved in freshly distilled thionyl chloride (250ml), N,N-dimethylformamide (DMF, 0.5ml) was added, and then heated to reflux for 3 hours. After the reaction was completed, it was allowed to cool naturally, and then the reactant was poured into ice water (1000ml), stirred continuously, then filtered, and dried to obtain a brownish-yellow solid. Under nitrogen protection, freshly distilled tetrahydrofuran (THF, 100ml) and sodium methoxide / methanol solution (25%, 30ml) were successively added to the brownish-yellow solid, and stirred at room temperature for 1 hour. Finish the reaction, add ethyl acetate (300ml) to the reactant, wash with water (200ml) three times, wash with saturated brine, dry over anhydrous sodium sulfate, filter, concentrate, column chromatography, use n-hexane as eluent, obtain Product 14.2 g, yield 98%.
[0038] 1 H NMR (300MHz, CDCl 3 ): δ7.58(1H, dd, J=2.1Hz, J=7.8Hz), 7.37-7.31(2H, m), 4.13(3H, s)...
Embodiment 3
[0040]
[0041] To the mixture of compound 3 (9g, 4.4mmol) and bromobutyrolactam (NBS, 7.83g, 4.4mmol), α, α-isobutylonitrile (a, a-azoisobutyronitrile, 715mg, 0.44mmol) Add 1,2-dichloroethane (200ml) to the mixture, and heat to reflux for 40 minutes under the irradiation of a 500-watt sun lamp. Cool naturally to room temperature, add silica gel (18 g, 60-230 μ), remove the solvent under reduced pressure, put the solid mixture on a silica gel column, rinse with n-hexane: ethyl acetate = 70: 1, and obtain 8.38 g of the product. The rate is 91%.
[0042] 1 HNMR (300MHz, CDCl 3 ): δ7.66(1Hmdd, J=1.5Hz, J=8.4Hz), 7.50(1H,dd, J=1.2Hz, J=6.9Hz), 7.39-7.34(1H, m), 4.96(2H, s), 4.12 (3H, s), 4.07 (3H, s) ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com