Acellular dermal matrix and its preparing method
A decellularized dermis and matrix technology, applied in skin transplantation, medical science, prosthetics, etc., can solve the problems of limited inactivation methods for mad cow disease pathogens, the risk of spreading viruses, and narrow sources of raw materials, etc., to achieve high practical application value and source Wide range of effects with good tissue compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, the preparation of acellular dermal matrix
[0044] Using sheep as the material source for preparing the acellular dermal matrix, the specific preparation process includes the following steps:
[0045] 1) peeling off the body surface skin of the sheep;
[0046] 2) Remove subcutaneous fat, muscle and hair and other incidental components;
[0047] 3) Wash the skin with clean water;
[0048] 4) Treat the skin with 2N sodium hydroxide solution for 40 minutes;
[0049] 5) replace with new 2N sodium hydroxide solution, and then treat for 40 minutes;
[0050] 6) Step 5) is repeated once;
[0051] 7) Treat the skin with 5% Tween-80 for 20 minutes;
[0052] 8) The skin treated by the above steps is fully washed with depyrogenated water to remove the residual chemical components to obtain the acellular dermal matrix, which is stored in PBS.
Embodiment 2
[0053] Embodiment 2, preparation of decellularized dermal matrix
[0054] Using cattle as the material source for preparing the acellular dermal matrix, the specific preparation process includes the following steps:
[0055] 1) peeling off the body surface skin of the cow;
[0056] 2) Remove subcutaneous fat, muscle and hair and other incidental components;
[0057] 3) Wash the skin with clean water;
[0058] 4) Treat the skin with 5N sodium hydroxide solution for 30 minutes;
[0059] 5) replace with new 5N sodium hydroxide solution, and then treat for 30 minutes;
[0060] 6) Step 5) is repeated twice;
[0061] 7) Treat the skin with 1% TRITON-X100 for 30 minutes;
[0062] 8) The skin treated by the above steps is fully washed with depyrogenated water to remove the residual chemical components to obtain the acellular dermal matrix, which is stored in PBS.
Embodiment 3
[0063] Example 3, Structural Observation and Detection of Acellular Dermal Matrix
[0064] 1. Observation of electron microscope structure of acellular dermal matrix
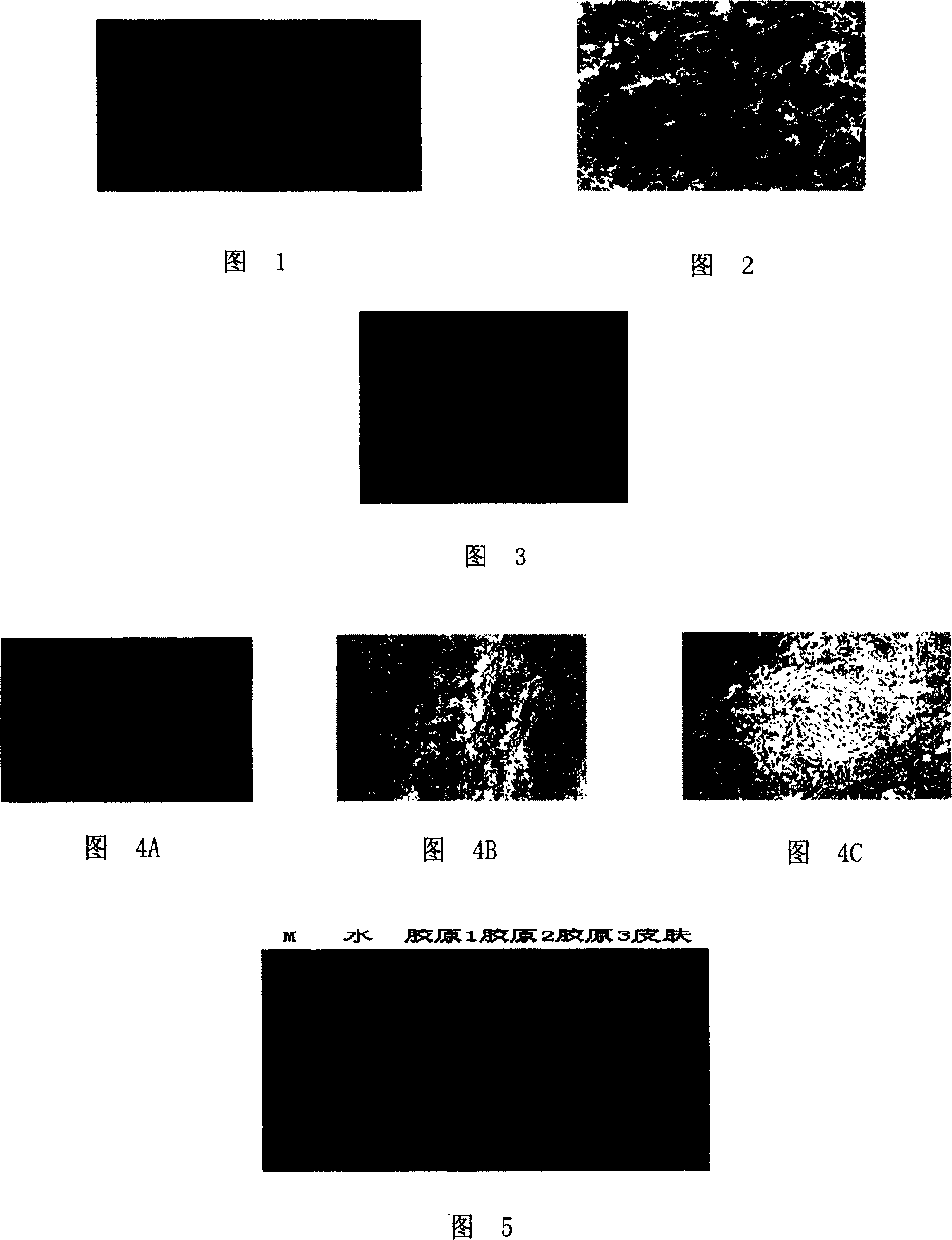
[0065] The freeze-dried photos of the acellular dermal matrix prepared in Example 1 and Example 2 are shown in Figure 1, and then its internal structure is observed with an electron microscope, as shown in Figure 2, indicating that the material space maintains a size of 20-100 microns The prepared acellular dermal matrix is suitable for the growth of various types of cells.
[0066] 2. Biocompatibility testing of acellular dermal matrix
[0067] Human fibroblasts were inoculated on the acellular dermal matrix prepared in Example 1 and Example 2, and the number of inoculated cells was 10 6 One was cultured at 37°C, and the growth of human fibroblasts was observed. The growth of the cells after inoculation was shown in Figure 3. The growth of human fibroblasts was vigorous, indicating that the prepared acellul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com