Immunoassay detection method for neonicotinyl insecticides
An immunoassay and pesticide technology, applied in the field of immunoassay to achieve the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
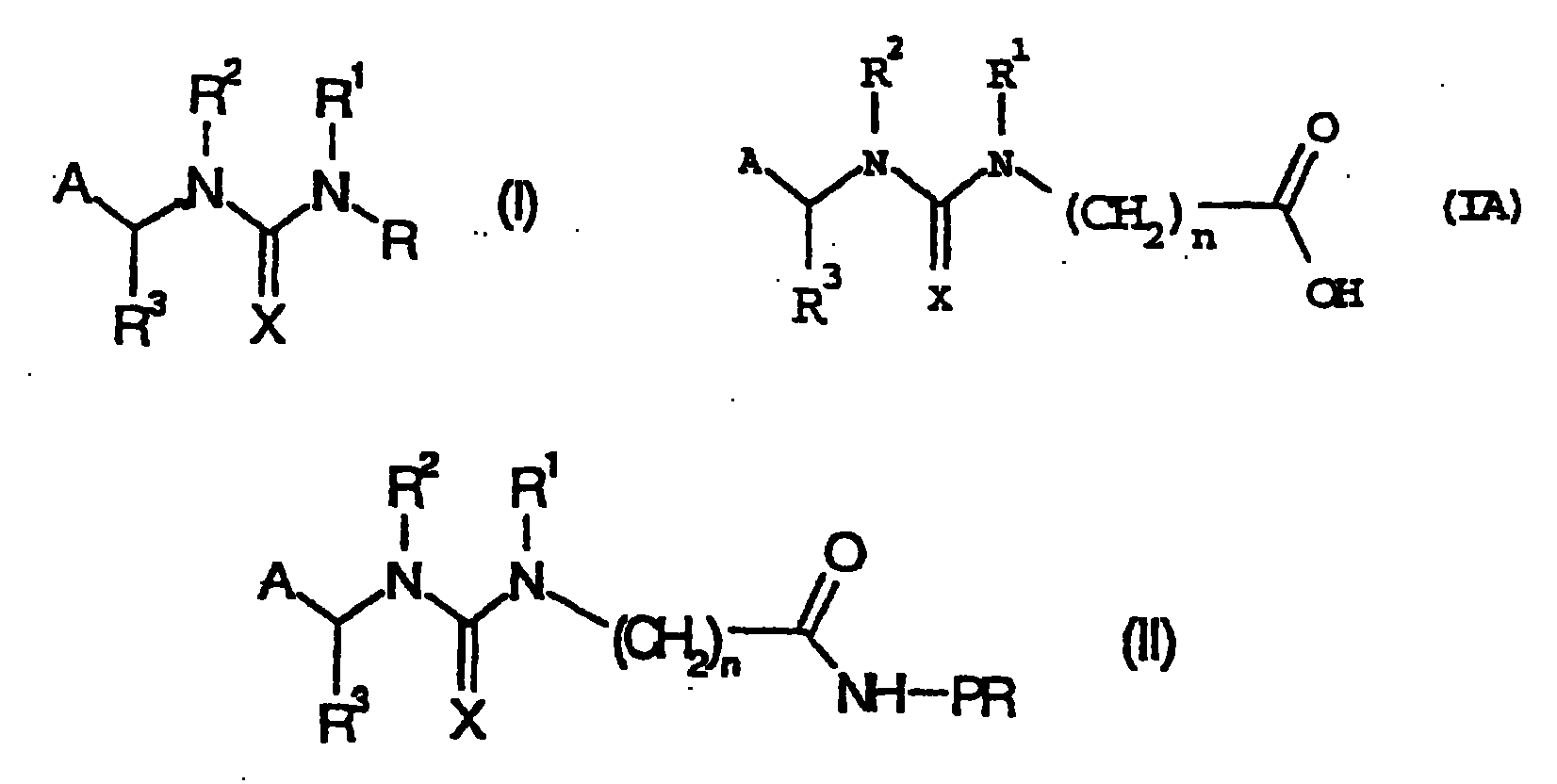
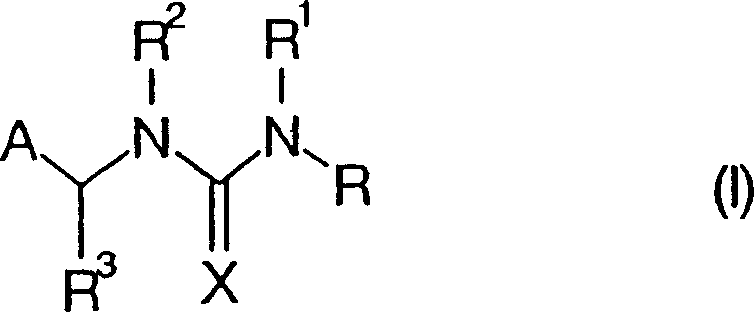
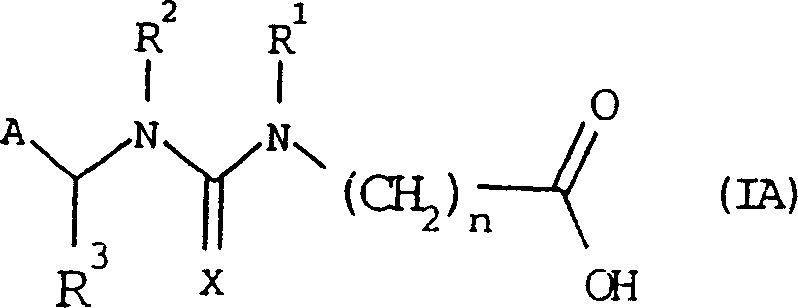
[0062] Example 1: Hapten (IIIA)
[0063]
[0064] where A, R 3 , X, and n have the same definitions as in general formula IA.
[0065] Hapten (IIIB)
[0066]
[0067] where A, R 3 , and X are defined the same as in general formula IA, and E is defined in the same way as in general formula IB.
[0068] Haptens of general formula (IIIA) and (IIIB) can be prepared methodologically analogously as follows:
[0069]
[0070] The desired haptens (IIIA) or (IIIB) are prepared using appropriate 2-aminoethanethiol derivatives selected from compounds having the following general formula:
[0071]
[0072] wherein R has the meanings given above for general formula (I).
[0073] The following haptens have been found to be particularly useful in the practice of the invention:
[0074]
[0075] Where n in each case is an integer of 1-5, preferably an integer of 3-5.
[0076] As the case may be, antigenic neonicotinoid conjugates (immunogens) can be generated and assayed...
Embodiment 1
[0093] Example 1: Synthetic process of thiamethoxam hapten
[0094]
[0095] 1.12-Methyl-1-nitroisourea
[0096] O-methylisourea sulfate (38.5 g) was dissolved in nitric acid (120 ml) / sulfuric acid (280 ml) mixture at 0°C, and stirred at 0°C for 2 hours. Carefully pour the mixture onto crushed ice and stir, then filter to remove the crushed ice. The needle-like crystals were dissolved in ethyl acetate, and potassium carbonate was added to make the solution alkaline. The solution was dried over magnesium sulfate and filtered. The filtrate was concentrated to give a white powder (5g).
[0097] 1.2
[0098] A solution was prepared from 3 g of methyl 4-aminobutyrate hydrochloride (1.1a) and 15 ml of water. The pH was adjusted to 11 by the dropwise addition of 2N NaOH while cooling the solution in an ice bath. The reaction flask was equipped with a pH electrode and a thermometer, and the resulting clear, alkaline solution was stirred rapidly while 2-methyl-1-nitroisourea (...
Embodiment 2-5
[0106] Examples 2-5: thiamethoxam haptens
[0107] The synthetic methodology of Example 1 was used to prepare the following thiamethoxam haptens shown in Table 1, wherein the haptens of the general formula NH 2 (CH 2 ) n CO 2 R(1.1b) (where n is an integer from 1 to 5, R is H or C 1 -C 4 Alkyl) appropriate analogues were carried out instead of intermediate 1.1a.
[0108] Table 1
[0109]
[0110] Example
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com