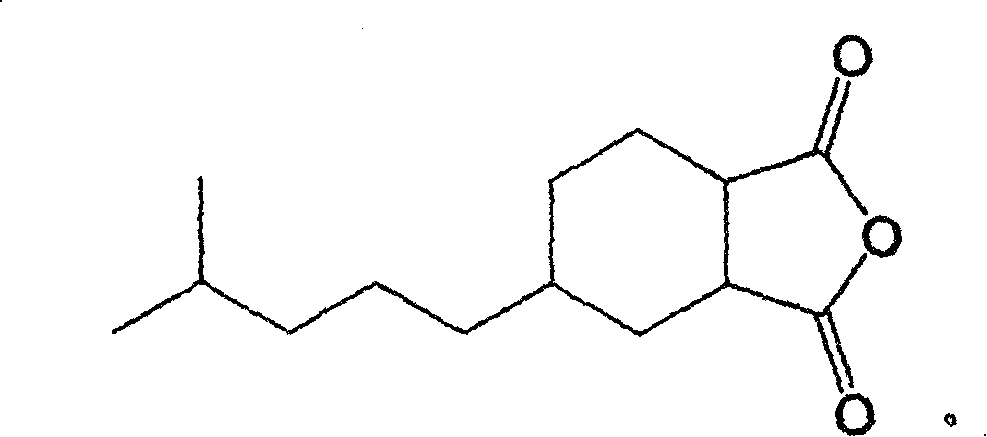
New use of 4-(4-mothyl-n-amyl) cyclohexane-1,2-diacid anhydride
A technology of methyl n-pentyl and cyclohexane, which is applied to the assembly of printed circuits, welding media, welding/cutting media/materials, etc. of electrical components, and can solve the problem of increased solder composition, complex additive structure, and complex composition, etc. problem, to achieve the effect of less residue, high soldering activity, and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
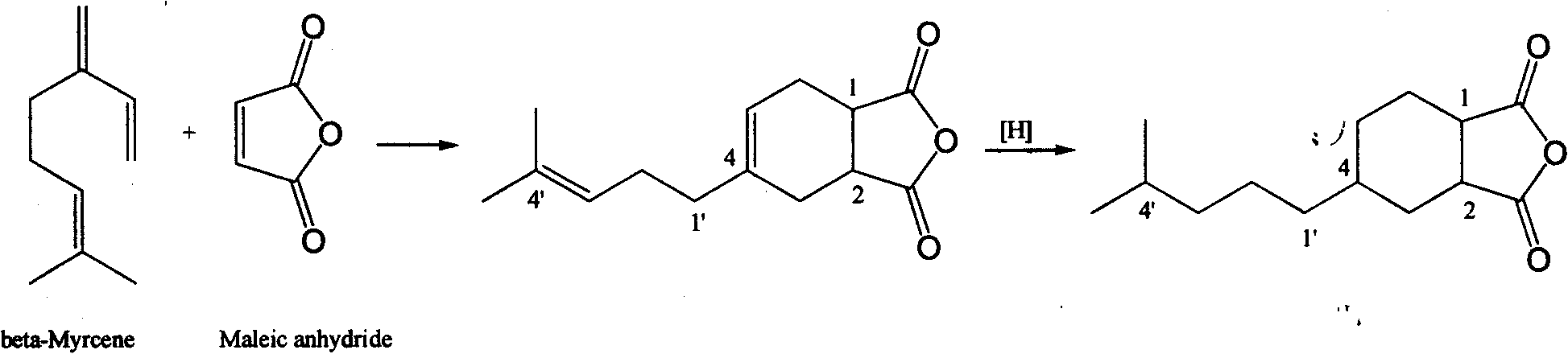
[0014] The specific preparation method is: using β-myrcene and maleic anhydride as raw materials, under heating conditions, after adding β-myrcene dropwise to maleic anhydride, carry out intermolecular Diels-Alder (Diels Alder) at the same temperature. -Alder) cycloaddition reaction at first obtains 4-(4'-methyl-3'-pentenyl)-4-cyclohexene-1,2-dioic anhydride cycloaddition product, then, it is dissolved in acetic acid In ethyl ester, with 5% Pd / C as catalyst, at room temperature and 1-5kg / cm 2 The hydrogenation reaction is carried out under hydrogen pressure to obtain the target 4-(4'-methyl-n-pentyl)-cyclohexane-1,2-dioic anhydride, whose structural feature is a cyclic monoterpene derivative with an anhydride functional group. After the hydrogenation reaction is completed, the catalyst, solvent and low boiling point components are removed to obtain the crude product, and the high-purity target product can be obtained by recrystallization method, and the welding activity test i...
Embodiment 1
[0018] Embodiment 1 (raw material 4-(4'-methyl-3'-pentenyl)-4-cyclohexene-1, the preparation of 2-dioic anhydride)
[0019] In a four-neck flask equipped with a thermometer, a dropping funnel, a mechanical stirrer, and a condenser, add maleic anhydride (calculated as 1.0 times the mole of β-myrcene) and heat to dissolve the maleic anhydride, controlled at 65- At 70°C, β-myrcene (75% content of β-myrcene) was added dropwise from the dropping funnel. After dropping, react at the same temperature for 4 hours. The lower fraction at 155-160°C / 3mmHg was collected by vacuum distillation to obtain the cycloaddition product 4-(4'-methyl-3'-pentenyl)-4-cyclohexene-1,2-dianhydride, which was analyzed by NMR Resonance determines structure.
Embodiment 2
[0020] Embodiment 2 (hydrogenation reaction)
[0021] The cycloaddition product 4-(4'-methyl-3'-pentenyl)-4-cyclohexene-1,2-dioic anhydride 32g obtained in Example 1 was dissolved in 97ml ethyl acetate, and the The ethyl acetate solution was added into the autoclave, and then 1.6 g of 5% Pd / C catalyst was weighed and added to the autoclave. After replacing the air in the kettle with hydrogen, fill the hydrogen to 5kg / cm 2 Then start the stirrer to adjust the stirring speed to 500rpm, the reaction starts immediately, the reactant starts to absorb hydrogen, and the hydrogen pressure drops. When the hydrogen pressure drops to 1kg / cm 2 Supplement hydrogen to 5kg / cm 2 , and repeat this operation. After the hydrogen absorption stopped, the catalyst was removed with a filter aid, and the solvent was evaporated with a rotary evaporator to obtain a crude hydrogenated product, which was used as solder A for the soldering test. Collect the fraction at 160-165°C / 1mmHg by vacuum disti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com