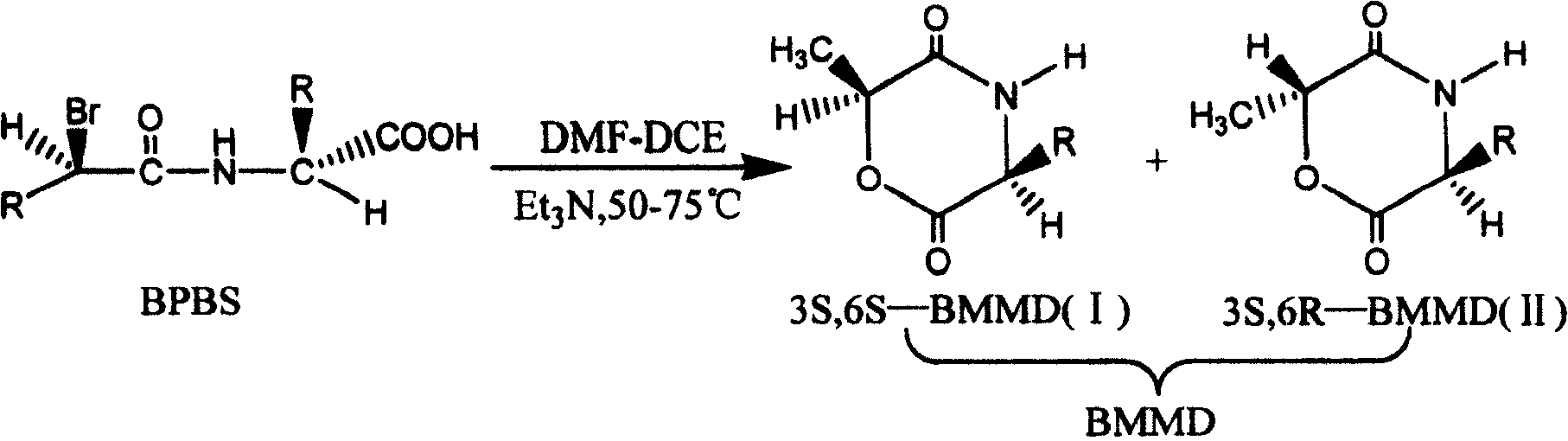
Preparation method of two kinds of pure optical isomerism serine morpholine dione
A serine morpholine dione, optical isomerization technology, applied in the direction of organic chemistry, can solve the problems of optical stereo racemization, resolution, preparation of pure optical isomers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 500ml of DMF-DCE mixture (volume ratio DMF / DCE=2.0) and 6.7ml of Et in a 1000ml reactor 3 N, heat the reaction kettle to 60±1°C, add BPBS DMF-DCE mixture (16.0g BPBS dissolved in 133ml DMF and 67ml DCE mixture) dropwise to the kettle under stirring, and finish adding in 2 hours at constant temperature at 60±1°C , and then react at a constant temperature of 60±1°C for 18 hours. After the reaction, the DMF-DCE mixed solution was evaporated under reduced pressure, and the solid was added to acetate (500ml of methyl acetate) to obtain a solution, which was transferred to a separatory funnel, washed with water (to remove the precipitate), and the water was discarded. After washing the oil phase with distilled water three times, the oil phase was dried with anhydrous magnesium sulfate for 24 hours. Then the solvent of the liquid phase is evaporated under reduced pressure, and then recrystallized with methyl acetate, and the product is dried at room temperature under vacu...
Embodiment 2
[0023] Add 550ml of DMF-DCE mixture (volume ratio DMF / DCE=9.0) and 7.3ml of Et in the 1000ml reactor 3 N, heat the reaction kettle to 60±1°C, add BPBS DMF-DCE mixture (17.5g BPBS dissolved in 180ml DMF and 20ml DCE mixture) dropwise to the kettle under stirring, and finish adding in 2 hours at constant temperature at 60±1°C , and then react at a constant temperature of 60±1°C for 18 hours. After the reaction, the DMF-DCE mixed solution was evaporated under reduced pressure, and the solid was added to acetate (500ml of propyl acetate) to obtain a solution, which was transferred to a separatory funnel, washed with water (to remove the precipitate), and the water was discarded. After washing the oil phase with distilled water three times, the oil phase was dried with anhydrous magnesium sulfate for 24 hours. Then the solvent of the liquid phase is evaporated under reduced pressure, and then recrystallized with propyl acetate, and the product is dried at room temperature under va...
Embodiment 3
[0025] Add 580ml of DMF-DCE mixture (volume ratio DMF / DCE=1.0) and 7.7ml of Et in a 1000ml reactor 3N, heat the reaction kettle to 55±1°C, add BPBS DMF-DCE mixture (18.6g BPBS dissolved in 100ml DMF and 100ml DCE mixture) dropwise to the kettle under stirring, and finish adding in 2 hours at a constant temperature of 55±1°C. Then react at a constant temperature of 55±1° C. for 18 hours. After the reaction, the DMF-DCE mixed solution was evaporated under reduced pressure, and the solid was added to acetate (tert-butyl acetate 500ml) to obtain a solution, which was transferred to a separatory funnel, washed with water (to remove the precipitate), and discarded. The water phase and the oil phase were washed three times with distilled water, and then dried over anhydrous magnesium sulfate for 24 hours. Then the solvent of the liquid phase is evaporated under reduced pressure, and then recrystallized with tert-butyl acetate, and the product is dried at room temperature under vacuu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com