Buckthorn extract, its preparing method and use in pharmaceutical process
A technology of extract and seabuckthorn, which is applied in the application field of preparation of medicines, to achieve the effect of increasing radiosensitivity, simple and practical method, and clear structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
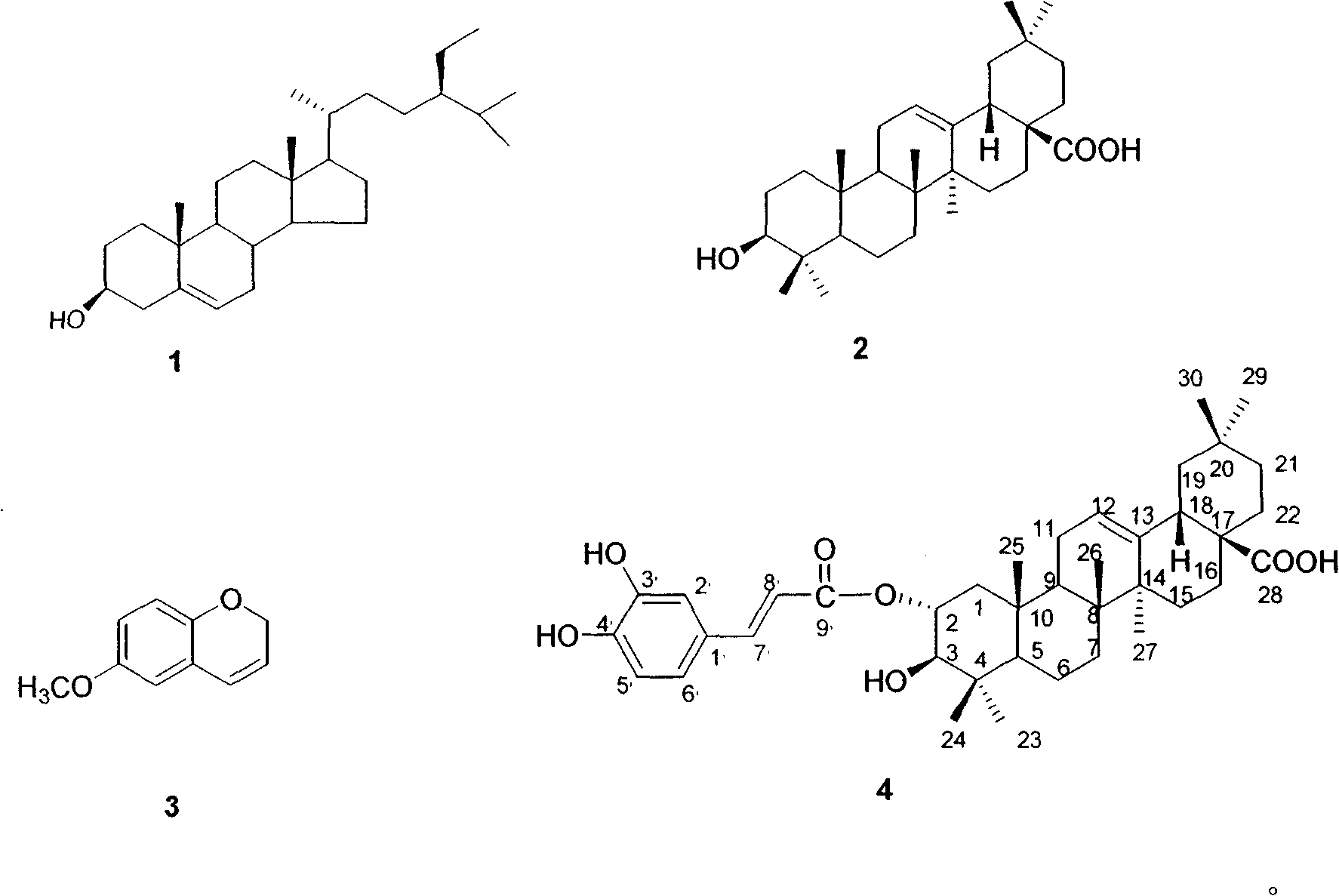
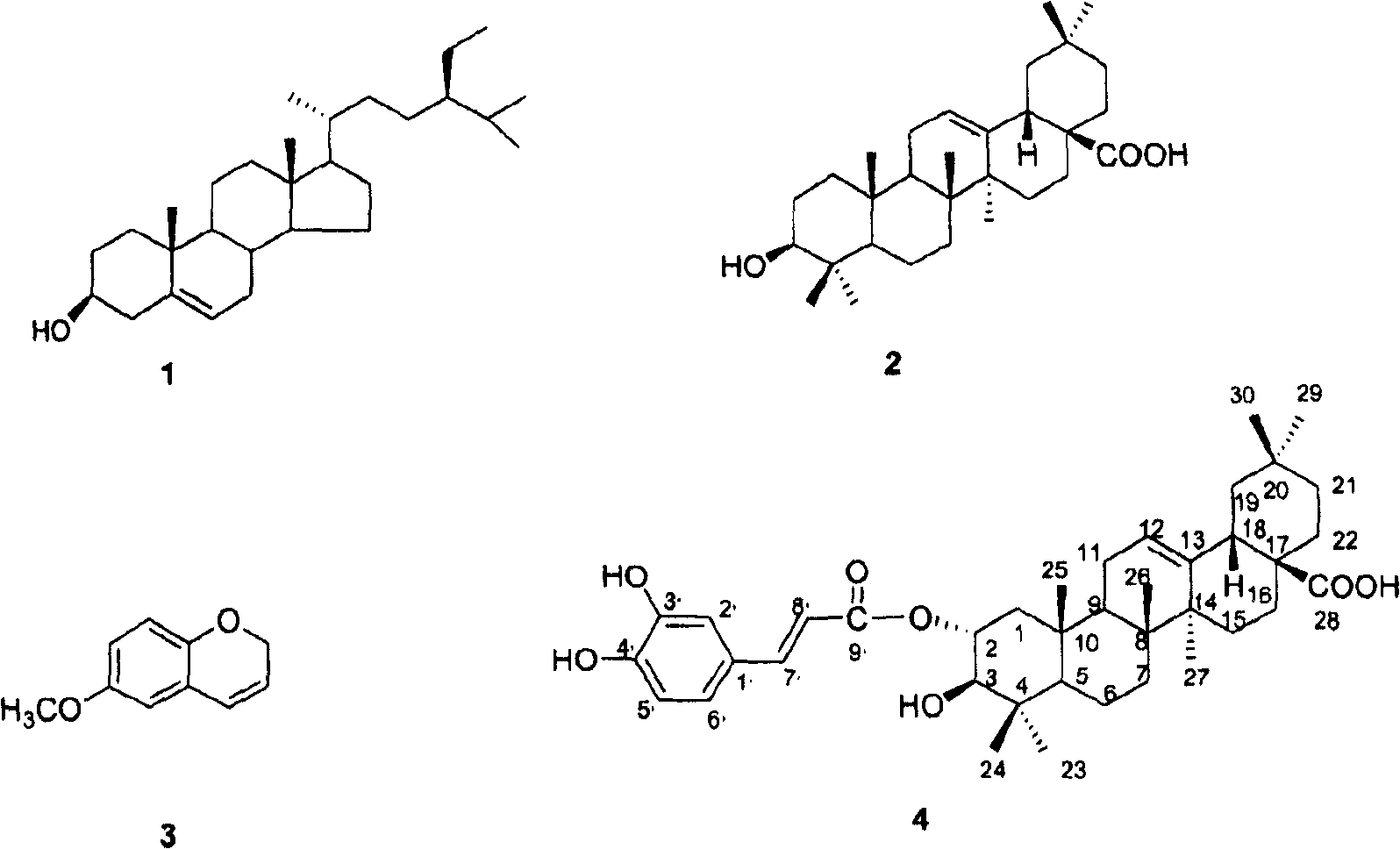
[0027] Example 1: Preparation of extract and determination of compound structure
[0028] 3.0 kg of Hippophae rhamnoides bark was crushed, 10 liters of 80% acetone (acetone: water = 8: 2) was added, and left at room temperature for 12 hours. Filter to obtain the extract. The extracted seabuckthorn powder was extracted again with 10 L of 80% acetone, and mixed with the previously obtained extract. This solution was concentrated under reduced pressure at 40°C to obtain 929.8 g of a brown powder extract.
[0029] Take 929.8g of powder extract and dissolve it in 3L of water, and then use 2L each of n-hexane, chloroform, ethyl acetate and butanol to extract in sequence. The extract was concentrated under reduced pressure at 40°C. The n-hexane layer obtained 21.14g of extract, the chloroform layer obtained 29.34g of extract, the ethyl acetate layer obtained 37.12g of extract, and the n-butanol layer obtained 180.23g of extract. The aqueous layer yielded 631.73 extracts. The NO p...
Embodiment 2
[0035] Embodiment 2: Determine the structure of physical and chemical parameters and compounds
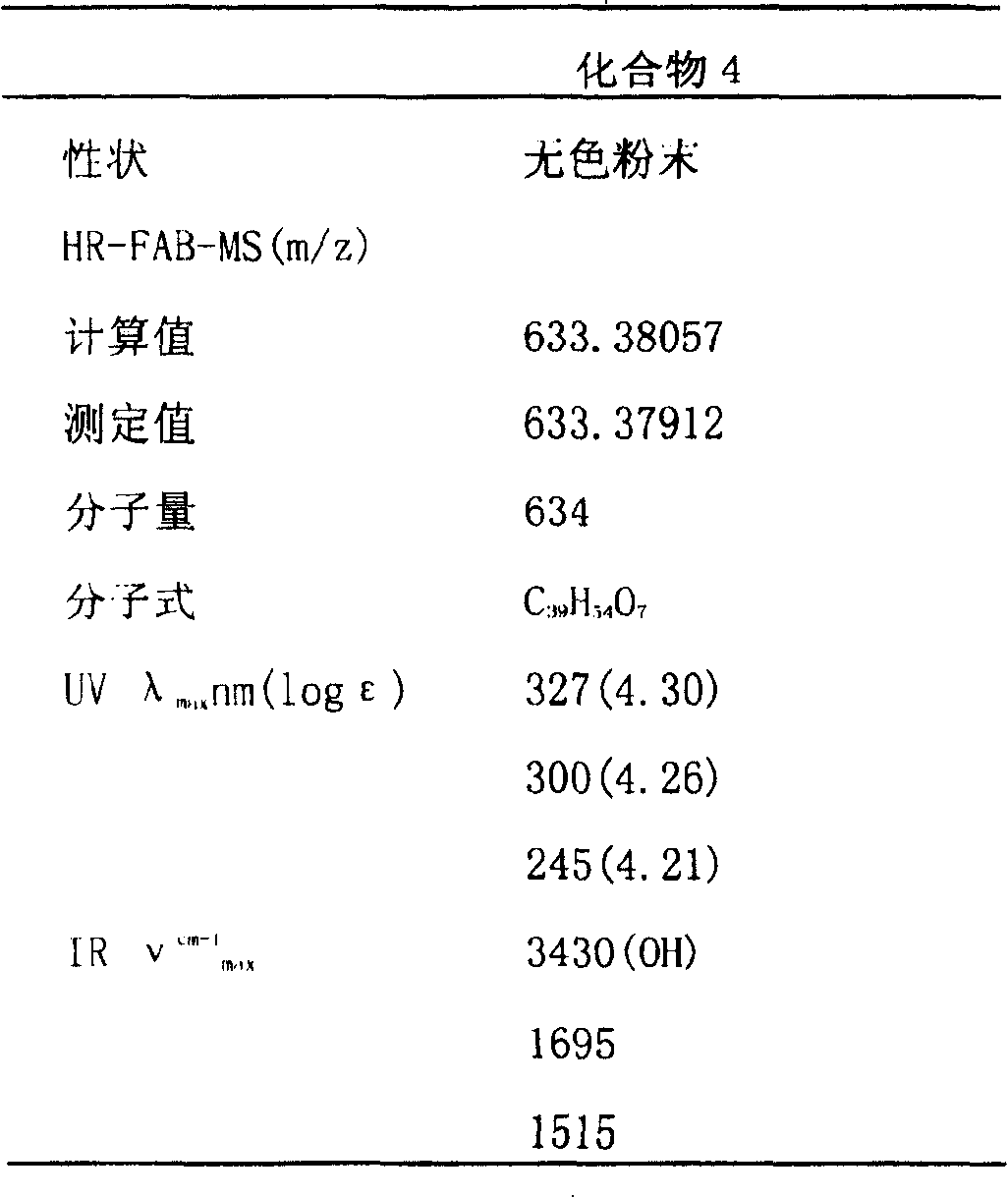
[0036] The character, molecular weight etc. of compound (4) are shown in Table 1; Compound (4) 13 C-NMR, 1 The H-NMR data are shown in Table 2. And finally determined the structural formula of the relevant compound by the above data.
[0037] Table 1: Properties, molecular weight, UV, IR of compound (4)
[0038]
[0039] The NMR spectral data (ppm, in Methanol-d) of table 2 compound 4 4 600MHz)
[0040] s: singlet d: doublet t: triplet m: multiplet
[0041] () value is Hz
[0042]
[0043] The structural formula of compound 4 is determined as follows:
[0044]
Embodiment 3
[0045] Embodiment 3: Inhibition of NO production test
[0046] The inhibitory effect on NO production (NO production inhibitory effect) of megalithic phagocytes stimulated by interferon-r and lipopolysaccharide was obtained by the following experimental method. And the inhibitory effect of NO production was evaluated based on the IC50(um) of the inhibitory effect.
[0047] Materials used:
[0048] RAW 264.7 cells (Dainippon Pharmaceutical Co., Ltd.)
[0049] N-1-naphthaleneethylenediamine hydrochloride (1g Wako Pure Chemical Industries)
[0050] Sulfa (500g Wako Pure Chemical Industries)
[0051] Ham's F12 Medium (SIGMA N488 500mL)
[0052] IFN-γ (Geneyme / Techne 100μg)
[0053] Lipopolysaccharide (LPS, 055:B5 10mg, Sigma)
[0054] Phosphoric acid (500ml Wako Pure Chemical Industries)
[0055] DMSO (500ml Wako Pure Chemical Industries)
[0056] 96-well microtiter plate (50 / box Sumitomo Bakelite, trade name [8096R])
[0057] experiment method:
[0058] RAW264.7 cells, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com