Shixiantao extract, its preparation method and its application in the preparation of medicine
A technology of fairy peach and medicine, applied in the field of Shixiantao extract, its preparation and its application in the preparation of medicines, to achieve the effects of increasing radiosensitivity, reducing tumor growth, and having no cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of extract and determination of compound structure
[0035]10.2Kg of the whole herb of Shixiantao was crushed, 55 liters of 70% ethanol (ethanol: water = 7: 3) was added, and left at room temperature for 12 hours. After about an hour of heating cycle, the extract is obtained. The extracted Shixiantao powder was extracted again with 55 L of 70% ethanol, and mixed with the extract obtained before. This solution was concentrated under reduced pressure at 40° C. to obtain 789.6 g of a brown powder extract.
[0036] Take 785.1g of the powder extract and dissolve it in 3L of water, then extract with 2L each of n-hexane, ethyl acetate and butanol in sequence. The extract of ethyl acetate was selected and concentrated under reduced pressure at 40° C. to obtain 220.1 g of brown powder.
[0037] Take 200.4 g of the ethyl acetate extract and separate it with a silica gel column (Wako gel C-300, 6.5×25 cm). Use n-hexane:ethyl acetate=100:0, 95:5, 90:10, 8...
Embodiment 2
[0041] Embodiment 2: Determine the structure of physical and chemical parameters and compounds
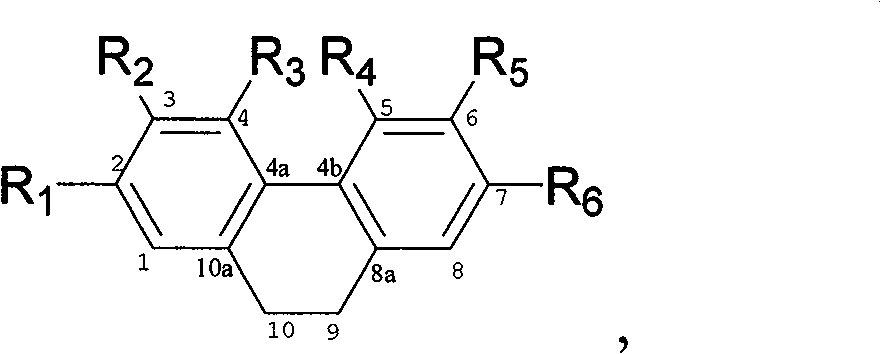
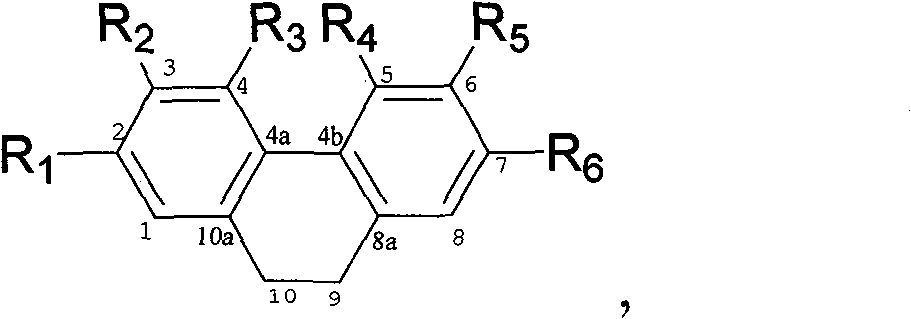
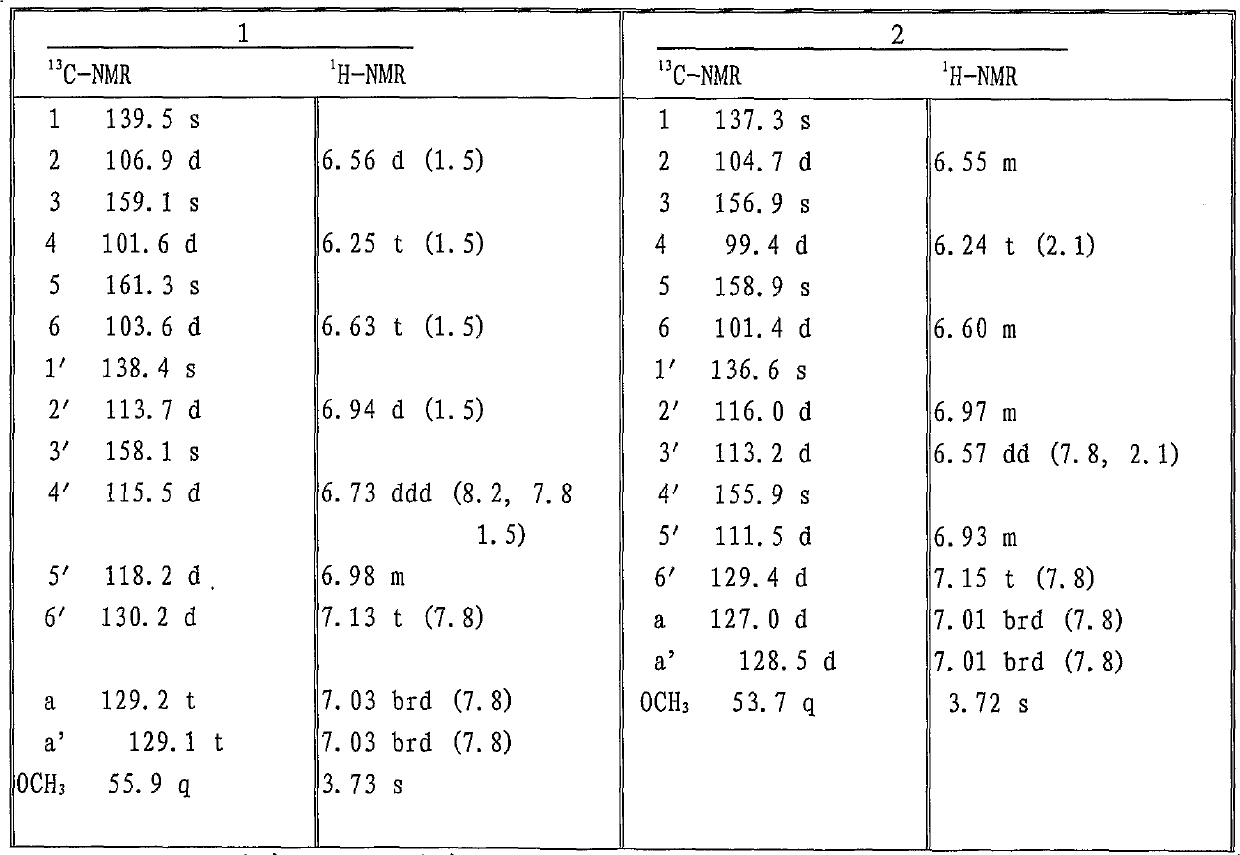
[0042] The properties and molecular weight of compounds (1)-(10) are shown in Table 1; compounds (1) and (2) 13 C-NMR, 1 The data of H-NMR is shown in Table 2, compound (3), (4), (5), (6), (7), (8), (9), (10) 13 C-NMR data are shown in Table 3, 1 H-NMR data are shown in Table 4.
[0043] And finally determined the structural formula of the relevant compound by the above data.
[0044] Table 1:
[0045] 1 2 3 4 5 Properties EI-MS(m / z) HR-EI-MS(m / z) Measured value Calculated value Molecular weight Molecular formula UVλ max MeOH nm(logε) IRV max KBr cm -1 Red Oil 242 242.09453 242.09428 242.1 C 15 h 14 o 3 208(4.40) 3311, 2929 2828, 1588 1493, 1193 1156, 966 Red Oil 242 242.09446 242.09443 242.1 C 15 h 14 o 3 208(4.42) 3389, 2935 2840, 1591 1454, 1153 1056, 957 White needle crystal 242 242.09430 242.09428 242.1 C 15 h ...
Embodiment 3
[0057] Embodiment 3: Inhibition of NO production test
[0058] The inhibitory effect on NO production (the effect of inhibiting NO production) of macrophages stimulated by interferon-r and lipopolysaccharide was determined by the following experimental method. And the inhibitory effect of NO production was evaluated based on the IC50 (μm) of the inhibitory effect.
[0059] Materials used:
[0060] RAW 264.7 cells (Dainippon Pharmaceutical Co., Ltd.)
[0061] N-1-naphthaleneethylenediamine hydrochloride (1g Wako Pure Chemical Industries)
[0062] Sulfa (500g Wako Pure Chemical Industries)
[0063] Ham's F12 Medium (SIGMA N488 500mL)
[0064] IFN-γ (Geneyme / Techne 100μg)
[0065] Lipopolysaccharide (LPS, 055:B5 10mg, Sigma)
[0066] Phosphoric acid (500ml Wako Pure Chemical Industries)
[0067] DMSO (500ml Wako Pure Chemical Industries)
[0068] 96-well microtiter plate (50 / box Sumitomo Bakelite, trade name [8096R])
[0069] experiment method:
[0070] RAW264.7 cells,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com