A fusion gene, it expressed protein and producing process thereof
A technology that fuses genes and proteins, applied in the fields of botanical equipment and methods, biochemical equipment and methods, genetic engineering, etc., to achieve the effect of relieving pain and the threat of infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] [Example 1] Construction of transfer plasmids pBac-CTBINB and pBI-CTBINB containing fusion genes
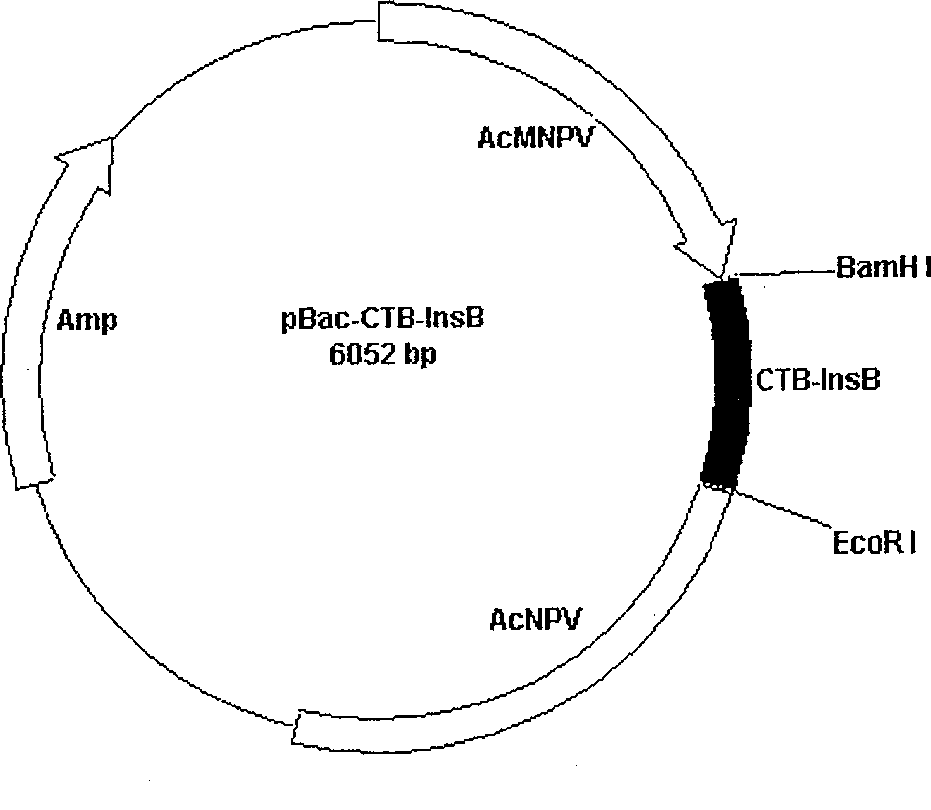
[0019] The pBac-CTBINS plasmid (see 200310121132.1 patent application specification) was used as a template and P1 and P2 were used as primers for amplification. The reaction conditions were pre-denaturation at 94°C for 5 minutes, amplification at 94°C for 1 minute, 57°C for 1 minute, and 72°C for 1 minute. Minutes, react for 30 cycles, and finally incubate at 72°C for 10 minutes to obtain the target fusion gene CTB-INB with a size of about 500 bp, and the 5' and 3' ends of the gene have BamHI and EcoRI sites respectively. The above-mentioned target fusion gene was digested with BamHI and EcoRI and connected to pBacPAK8 (CLONTECH Company) which was also digested with BamHI and EcoRI to construct the bacmid transfer plasmid pBac-CTBINB containing CTB and human insulin B chain gene. After enzyme digestion analysis and PCR identified the correct insertion of the gene, automat...
Embodiment 2
[0020] [Example 2] Obtaining of recombinant baculovirus of fusion gene of CTB and human insulin B chain
[0021] Take 5ul insect baculovirus transfer plasmid pBac-CTBINB containing CTB and human insulin B-chain fusion gene and 6ul wild silkworm nuclear polyhedrosis virus DNA for co-transfection. Take 6ul Lipofectin (GIBCOBRL company) and add 100ul serum-free TC-100 medium and mix well. The BmN cells previously cultured in a 35mm Dish were washed twice with serum-free TC-100 (GIBCOBRL company) medium, and the transfer plasmid and Lipofectin mixture was added dropwise, cultured at 27°C for 4-5 days, and the supernatant was collected for the second stage. One round of plaque screening. Take 5ul of the supernatant to infect the BmN cells in a 35mm Dish, discard the supernatant after 1 hour and add an equal amount of mixed TC-100 medium and low melting point agarose. Pick plaques after 4-5 days, infect BmN cells for 3-4 days, save the supernatant, lyse the cells with NaOH for Sou...
Embodiment 3
[0022] [Example 3] Expression of CTB and human insulin B chain fusion gene in silkworm larvae and pupae
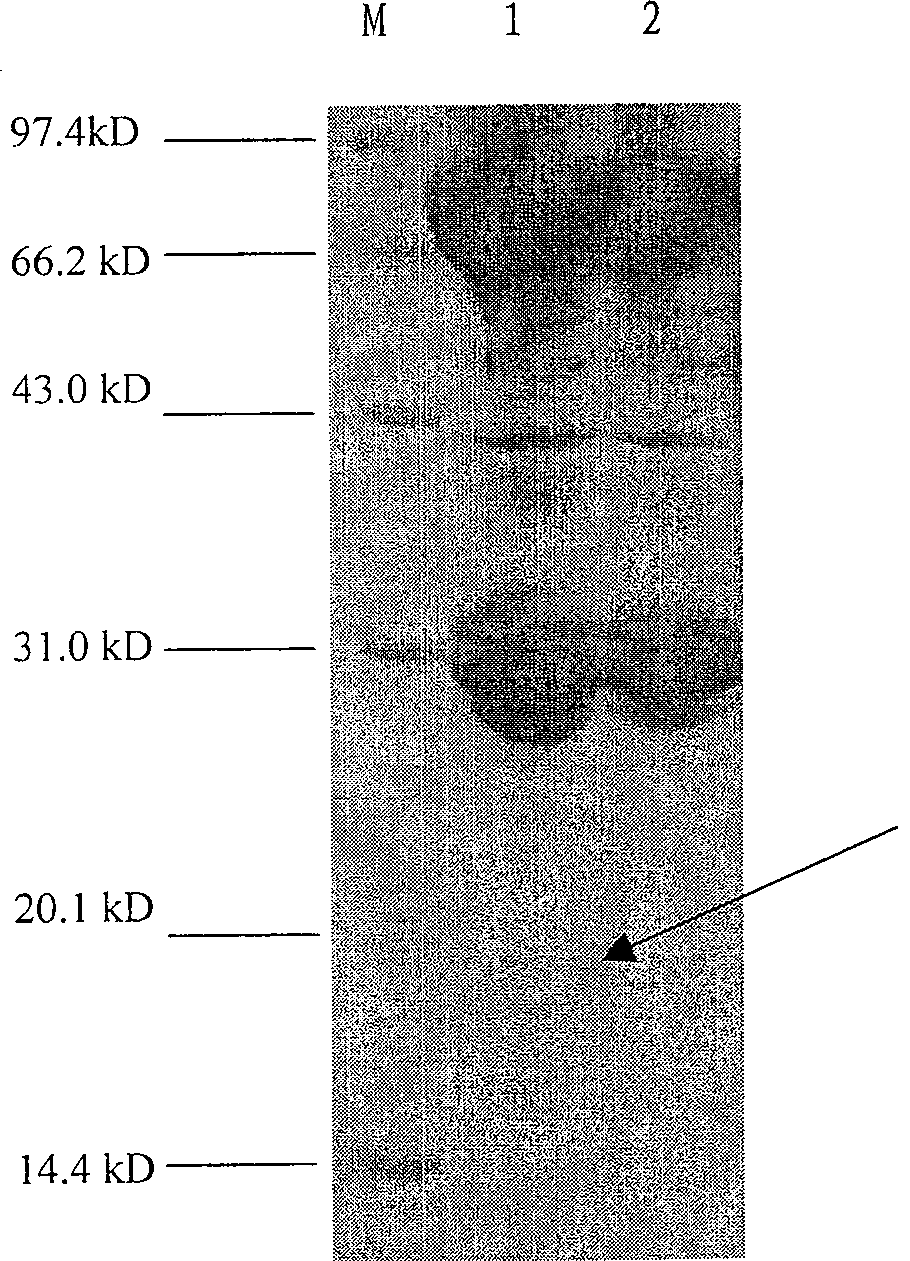
[0023] Take the amplified recombinant baculovirus containing fusion gene of CTB and human insulin B chain and inject it into fifth instar silkworm (Bombyx mori) larvae and pupae, inject about 2ul per head (titer is 1×10 7 / ml), take the silkworm lymph and pupal blood expressed at 24, 48, 72, 96, 120 and 144 hours respectively, centrifuge at 5000rpm for 5min, take the supernatant, dilute 10 times with PBS pH7.4, add an equal volume of 2× Protein loading buffer (100Mm Tris.HCl, 4% SDS, 0.1% bromophenol blue, 10% glycerol), take 20ul for SDS-PAGE analysis, see appendix figure 2 . The results showed that fusion protein of CTB and human insulin B chain was highly expressed in silkworm and pupae, and Western hybridization (attached image 3 ) shows that the molecular weight of the expression product is 18kD. The hemolymph of silkworm larvae and pupal bodies containing the fu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com